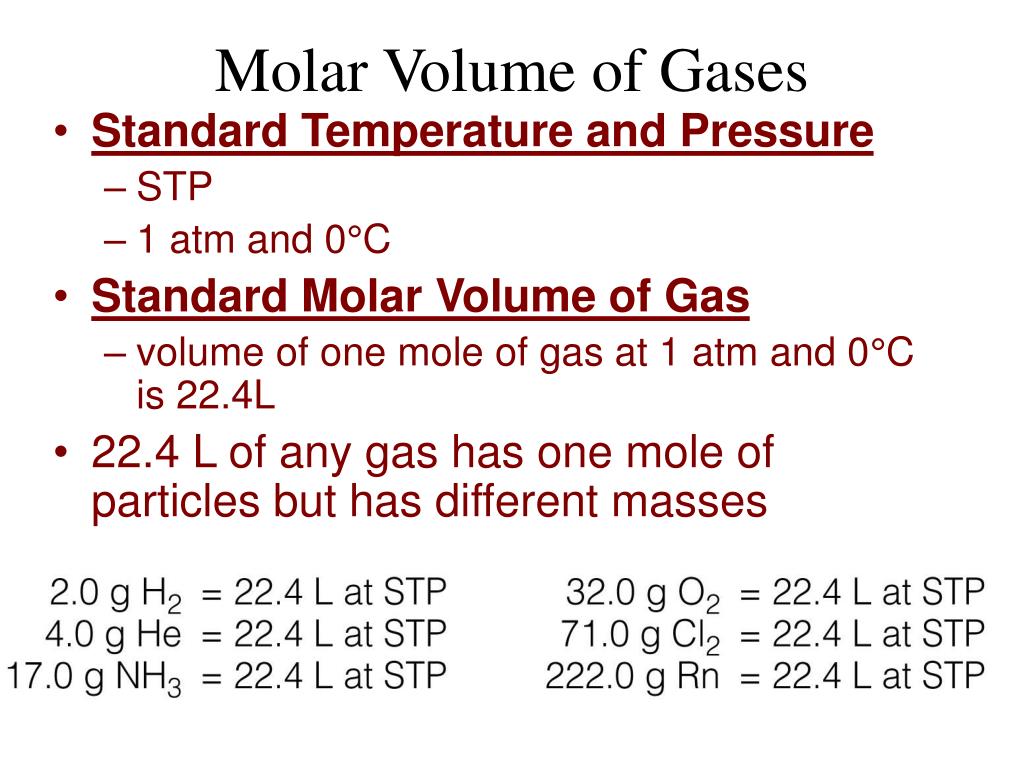
Gas Analysis Based On The Molar Volume . Plot a graph of mass of calcium carbonate (x) against volume. This allowed calculation of the molar volume of oxygen as 24.2. Record your results in a suitable way. The volume of gas produced in a reaction can be measured by collecting the gas with a gas. By analyzing the composition of natural gas, one can adjust the ideal gas law to account for these interactions and properly calculate the. To put everything together as you analyze your data to confirm avogadro’s hypothesis, you will use the combined ideal gas law to calculate. The gas volume, temperature, and pressure were measured and used to calculate the moles of oxygen. Using the ideal gas law and assuming standard pressure and temperature (stp), the volume of one mole of gas can be calculated: Measuring the molar volume of a gas. The volume (\(v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t). All of the empirical gas relationships are special cases of. The volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume.
from www.slideserve.com
Using the ideal gas law and assuming standard pressure and temperature (stp), the volume of one mole of gas can be calculated: The volume of gas produced in a reaction can be measured by collecting the gas with a gas. The volume (\(v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t). This allowed calculation of the molar volume of oxygen as 24.2. All of the empirical gas relationships are special cases of. The volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. Record your results in a suitable way. Measuring the molar volume of a gas. The gas volume, temperature, and pressure were measured and used to calculate the moles of oxygen. Plot a graph of mass of calcium carbonate (x) against volume.
PPT Lesson 2 The Molar Relationships PowerPoint Presentation, free
Gas Analysis Based On The Molar Volume This allowed calculation of the molar volume of oxygen as 24.2. This allowed calculation of the molar volume of oxygen as 24.2. All of the empirical gas relationships are special cases of. Using the ideal gas law and assuming standard pressure and temperature (stp), the volume of one mole of gas can be calculated: The volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. To put everything together as you analyze your data to confirm avogadro’s hypothesis, you will use the combined ideal gas law to calculate. Measuring the molar volume of a gas. By analyzing the composition of natural gas, one can adjust the ideal gas law to account for these interactions and properly calculate the. Record your results in a suitable way. The volume of gas produced in a reaction can be measured by collecting the gas with a gas. Plot a graph of mass of calcium carbonate (x) against volume. The volume (\(v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t). The gas volume, temperature, and pressure were measured and used to calculate the moles of oxygen.
From www.slideserve.com
PPT Physical Characteristics of Gases PowerPoint Presentation, free Gas Analysis Based On The Molar Volume The volume (\(v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t). The volume of gas produced in a reaction can be measured by collecting the gas with a gas. Plot a graph of mass of calcium carbonate (x) against volume. This allowed calculation. Gas Analysis Based On The Molar Volume.
From chemistrymadesimple.net
Molar Volume of Gases What It Is and How To Use It Chemistry Made Gas Analysis Based On The Molar Volume All of the empirical gas relationships are special cases of. Using the ideal gas law and assuming standard pressure and temperature (stp), the volume of one mole of gas can be calculated: The volume (\(v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t).. Gas Analysis Based On The Molar Volume.
From www.youtube.com
CHEMISTRY 101 Density of a gas, molar volume, and molar mass YouTube Gas Analysis Based On The Molar Volume This allowed calculation of the molar volume of oxygen as 24.2. To put everything together as you analyze your data to confirm avogadro’s hypothesis, you will use the combined ideal gas law to calculate. Plot a graph of mass of calcium carbonate (x) against volume. Record your results in a suitable way. The volume (\(v\)) of an ideal gas varies. Gas Analysis Based On The Molar Volume.
From studylib.net
Determination of the Molar Volume of a Gas Tri Gas Analysis Based On The Molar Volume Record your results in a suitable way. Measuring the molar volume of a gas. The volume (\(v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t). By analyzing the composition of natural gas, one can adjust the ideal gas law to account for these. Gas Analysis Based On The Molar Volume.
From www.numerade.com
Calculate the density of oxygen gas at STP using the molar volume of Gas Analysis Based On The Molar Volume All of the empirical gas relationships are special cases of. Record your results in a suitable way. Measuring the molar volume of a gas. By analyzing the composition of natural gas, one can adjust the ideal gas law to account for these interactions and properly calculate the. The volume (\(v\)) of an ideal gas varies directly with the number of. Gas Analysis Based On The Molar Volume.
From www.slideserve.com
PPT Chapter 14 Gases PowerPoint Presentation, free download ID Gas Analysis Based On The Molar Volume Using the ideal gas law and assuming standard pressure and temperature (stp), the volume of one mole of gas can be calculated: To put everything together as you analyze your data to confirm avogadro’s hypothesis, you will use the combined ideal gas law to calculate. Measuring the molar volume of a gas. Plot a graph of mass of calcium carbonate. Gas Analysis Based On The Molar Volume.
From www.researchgate.net
The molar volume of CO 2 computed by RedlichKwong equation (line) and Gas Analysis Based On The Molar Volume The volume of gas produced in a reaction can be measured by collecting the gas with a gas. Measuring the molar volume of a gas. This allowed calculation of the molar volume of oxygen as 24.2. Using the ideal gas law and assuming standard pressure and temperature (stp), the volume of one mole of gas can be calculated: Record your. Gas Analysis Based On The Molar Volume.
From www.youtube.com
How to Calculate Molar Volume of Gases and Reacting Gas Volume ratios Gas Analysis Based On The Molar Volume The volume (\(v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t). All of the empirical gas relationships are special cases of. Plot a graph of mass of calcium carbonate (x) against volume. To put everything together as you analyze your data to confirm. Gas Analysis Based On The Molar Volume.
From www.youtube.com
Molar Volume Calculated Two Different Ways YouTube Gas Analysis Based On The Molar Volume Plot a graph of mass of calcium carbonate (x) against volume. The volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. The gas volume, temperature, and pressure were measured and used to calculate the moles of oxygen. Record your results in a suitable way. The volume of gas produced in a reaction. Gas Analysis Based On The Molar Volume.
From mavink.com
How To Calculate Molar Volume Of A Gas Gas Analysis Based On The Molar Volume To put everything together as you analyze your data to confirm avogadro’s hypothesis, you will use the combined ideal gas law to calculate. By analyzing the composition of natural gas, one can adjust the ideal gas law to account for these interactions and properly calculate the. The volume of 1 mol of an ideal gas at stp is 22.41 l,. Gas Analysis Based On The Molar Volume.
From www.pc.maricopa.edu
Molar Volume of a Gas Gas Analysis Based On The Molar Volume The volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. Measuring the molar volume of a gas. The volume (\(v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t). Record your results in a suitable way.. Gas Analysis Based On The Molar Volume.
From www.youtube.com
Molar Volume of a Gas Lab Part 1 YouTube Gas Analysis Based On The Molar Volume By analyzing the composition of natural gas, one can adjust the ideal gas law to account for these interactions and properly calculate the. Record your results in a suitable way. The volume of gas produced in a reaction can be measured by collecting the gas with a gas. The volume (\(v\)) of an ideal gas varies directly with the number. Gas Analysis Based On The Molar Volume.
From www.youtube.com
5.5 Application of the Ideal Gas Law Molar Volumes, Density, & Molar Gas Analysis Based On The Molar Volume This allowed calculation of the molar volume of oxygen as 24.2. Measuring the molar volume of a gas. The volume of gas produced in a reaction can be measured by collecting the gas with a gas. Using the ideal gas law and assuming standard pressure and temperature (stp), the volume of one mole of gas can be calculated: By analyzing. Gas Analysis Based On The Molar Volume.
From www.slideserve.com
PPT Lesson 2 The Molar Relationships PowerPoint Presentation, free Gas Analysis Based On The Molar Volume The volume of gas produced in a reaction can be measured by collecting the gas with a gas. Plot a graph of mass of calcium carbonate (x) against volume. The volume (\(v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t). This allowed calculation. Gas Analysis Based On The Molar Volume.
From www.chegg.com
Solved 6 Gas Analysis Based on the Molar Volume FUNDAMENTAL Gas Analysis Based On The Molar Volume Record your results in a suitable way. The volume of gas produced in a reaction can be measured by collecting the gas with a gas. Measuring the molar volume of a gas. Using the ideal gas law and assuming standard pressure and temperature (stp), the volume of one mole of gas can be calculated: This allowed calculation of the molar. Gas Analysis Based On The Molar Volume.
From studylib.net
The Molar Volume of a Gas Gas Analysis Based On The Molar Volume The volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. The gas volume, temperature, and pressure were measured and used to calculate the moles of oxygen. By analyzing the composition of natural gas, one can adjust the ideal gas law to account for these interactions and properly calculate the. Using the ideal. Gas Analysis Based On The Molar Volume.
From www.slideserve.com
PPT Molar Volume and Ideal Gas Law PowerPoint Presentation, free Gas Analysis Based On The Molar Volume By analyzing the composition of natural gas, one can adjust the ideal gas law to account for these interactions and properly calculate the. Using the ideal gas law and assuming standard pressure and temperature (stp), the volume of one mole of gas can be calculated: Measuring the molar volume of a gas. The volume (\(v\)) of an ideal gas varies. Gas Analysis Based On The Molar Volume.
From www.youtube.com
Chapter 38 molar volume of gas YouTube Gas Analysis Based On The Molar Volume To put everything together as you analyze your data to confirm avogadro’s hypothesis, you will use the combined ideal gas law to calculate. By analyzing the composition of natural gas, one can adjust the ideal gas law to account for these interactions and properly calculate the. The volume of 1 mol of an ideal gas at stp is 22.41 l,. Gas Analysis Based On The Molar Volume.
From www.nagwa.com
Lesson Video Standard Molar Gas Volumes Nagwa Gas Analysis Based On The Molar Volume The gas volume, temperature, and pressure were measured and used to calculate the moles of oxygen. The volume (\(v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t). Record your results in a suitable way. Plot a graph of mass of calcium carbonate (x). Gas Analysis Based On The Molar Volume.
From www.youtube.com
Summary Molar Volume Calculations YouTube Gas Analysis Based On The Molar Volume To put everything together as you analyze your data to confirm avogadro’s hypothesis, you will use the combined ideal gas law to calculate. Plot a graph of mass of calcium carbonate (x) against volume. The volume of gas produced in a reaction can be measured by collecting the gas with a gas. Using the ideal gas law and assuming standard. Gas Analysis Based On The Molar Volume.
From www.slideserve.com
PPT Molar Volume of a Gas LAB PowerPoint Presentation, free download Gas Analysis Based On The Molar Volume Using the ideal gas law and assuming standard pressure and temperature (stp), the volume of one mole of gas can be calculated: The gas volume, temperature, and pressure were measured and used to calculate the moles of oxygen. This allowed calculation of the molar volume of oxygen as 24.2. By analyzing the composition of natural gas, one can adjust the. Gas Analysis Based On The Molar Volume.
From www.slideserve.com
PPT Molar Volume of a Gas PowerPoint Presentation, free download ID Gas Analysis Based On The Molar Volume Measuring the molar volume of a gas. Using the ideal gas law and assuming standard pressure and temperature (stp), the volume of one mole of gas can be calculated: This allowed calculation of the molar volume of oxygen as 24.2. Plot a graph of mass of calcium carbonate (x) against volume. All of the empirical gas relationships are special cases. Gas Analysis Based On The Molar Volume.
From centraltutors.co.uk
How to solve Mole Calculations using Molar Volume Central Tutors Gas Analysis Based On The Molar Volume Using the ideal gas law and assuming standard pressure and temperature (stp), the volume of one mole of gas can be calculated: This allowed calculation of the molar volume of oxygen as 24.2. By analyzing the composition of natural gas, one can adjust the ideal gas law to account for these interactions and properly calculate the. The volume (\(v\)) of. Gas Analysis Based On The Molar Volume.
From www.youtube.com
Molar Volume of Gases Examples The Mole Concept YouTube Gas Analysis Based On The Molar Volume To put everything together as you analyze your data to confirm avogadro’s hypothesis, you will use the combined ideal gas law to calculate. Using the ideal gas law and assuming standard pressure and temperature (stp), the volume of one mole of gas can be calculated: Plot a graph of mass of calcium carbonate (x) against volume. The gas volume, temperature,. Gas Analysis Based On The Molar Volume.
From www.youtube.com
Molar Volume of Gases The Mole Concept YouTube Gas Analysis Based On The Molar Volume The volume (\(v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t). Plot a graph of mass of calcium carbonate (x) against volume. By analyzing the composition of natural gas, one can adjust the ideal gas law to account for these interactions and properly. Gas Analysis Based On The Molar Volume.
From users.highland.edu
Molar Volume of a Gas Gas Analysis Based On The Molar Volume Using the ideal gas law and assuming standard pressure and temperature (stp), the volume of one mole of gas can be calculated: Record your results in a suitable way. To put everything together as you analyze your data to confirm avogadro’s hypothesis, you will use the combined ideal gas law to calculate. Measuring the molar volume of a gas. All. Gas Analysis Based On The Molar Volume.
From es.scribd.com
05 Determining the Molar Volume of a Gas Mol (Unidad) Magnesio Gas Analysis Based On The Molar Volume Measuring the molar volume of a gas. To put everything together as you analyze your data to confirm avogadro’s hypothesis, you will use the combined ideal gas law to calculate. Plot a graph of mass of calcium carbonate (x) against volume. By analyzing the composition of natural gas, one can adjust the ideal gas law to account for these interactions. Gas Analysis Based On The Molar Volume.
From www.youtube.com
Molar Volume Of Gas Chemical Calculations Chemistry FuseSchool Gas Analysis Based On The Molar Volume The volume of gas produced in a reaction can be measured by collecting the gas with a gas. Plot a graph of mass of calcium carbonate (x) against volume. This allowed calculation of the molar volume of oxygen as 24.2. Using the ideal gas law and assuming standard pressure and temperature (stp), the volume of one mole of gas can. Gas Analysis Based On The Molar Volume.
From www.tes.com
Molar Volume of Gases GCSE Lesson (SC14e) TRIPLE Teaching Resources Gas Analysis Based On The Molar Volume This allowed calculation of the molar volume of oxygen as 24.2. To put everything together as you analyze your data to confirm avogadro’s hypothesis, you will use the combined ideal gas law to calculate. Record your results in a suitable way. Using the ideal gas law and assuming standard pressure and temperature (stp), the volume of one mole of gas. Gas Analysis Based On The Molar Volume.
From www.slideserve.com
PPT Gases PowerPoint Presentation, free download ID436454 Gas Analysis Based On The Molar Volume The volume (\(v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t). Using the ideal gas law and assuming standard pressure and temperature (stp), the volume of one mole of gas can be calculated: To put everything together as you analyze your data to. Gas Analysis Based On The Molar Volume.
From www.chegg.com
Solved The Molar Volume of a Gas In this experiment, you Gas Analysis Based On The Molar Volume The volume (\(v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t). The gas volume, temperature, and pressure were measured and used to calculate the moles of oxygen. The volume of gas produced in a reaction can be measured by collecting the gas with. Gas Analysis Based On The Molar Volume.
From www.slideserve.com
PPT Molar Volume and Ideal Gas Law PowerPoint Presentation, free Gas Analysis Based On The Molar Volume The volume of gas produced in a reaction can be measured by collecting the gas with a gas. All of the empirical gas relationships are special cases of. The volume (\(v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t). Using the ideal gas. Gas Analysis Based On The Molar Volume.
From www.studocu.com
The Molar Volume of a Gas Introduction Based on the reaction of Gas Analysis Based On The Molar Volume The gas volume, temperature, and pressure were measured and used to calculate the moles of oxygen. All of the empirical gas relationships are special cases of. The volume (\(v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t). Plot a graph of mass of. Gas Analysis Based On The Molar Volume.
From www.youtube.com
MOLAR VOLUME OF A GAS PreLab NYA General Chemistry YouTube Gas Analysis Based On The Molar Volume To put everything together as you analyze your data to confirm avogadro’s hypothesis, you will use the combined ideal gas law to calculate. This allowed calculation of the molar volume of oxygen as 24.2. The volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. The volume of gas produced in a reaction. Gas Analysis Based On The Molar Volume.
From slideplayer.com
Molar Volume of a Gas Lab ppt download Gas Analysis Based On The Molar Volume The volume (\(v\)) of an ideal gas varies directly with the number of moles of the gas (n) when the pressure (p) and the number of temperature (t). Record your results in a suitable way. The gas volume, temperature, and pressure were measured and used to calculate the moles of oxygen. To put everything together as you analyze your data. Gas Analysis Based On The Molar Volume.