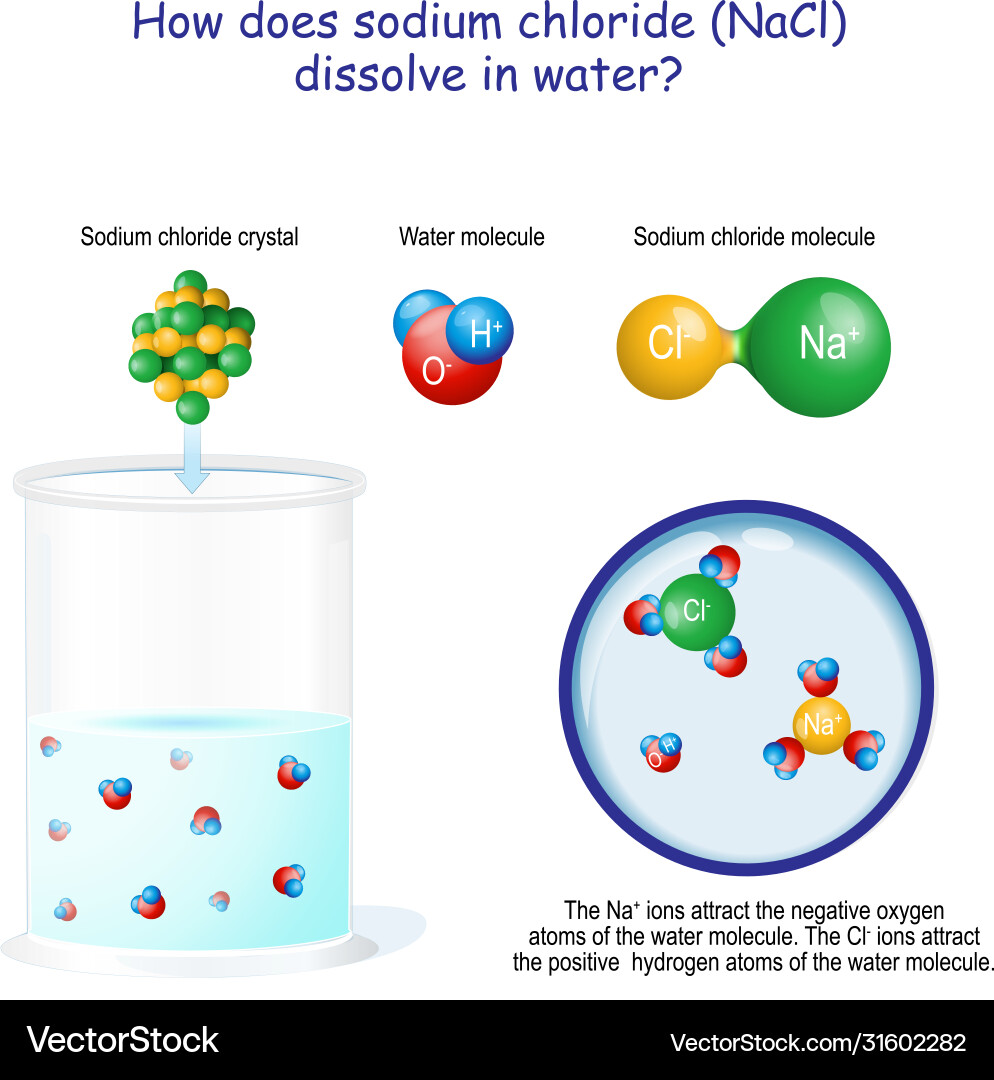
Why Does Table Salt Dissolve In Water But Not Cooking Oil . We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Nonpolar molecules such as those found in grease or oil do not dissolve in water. Why does salt dissolve in water but not in oil? When salt is added to water, the polar water molecules surround the charged ions (sodium and chloride), pulling them away from each other and dispersing them evenly throughout the solution. We will first examine the process that occurs when an ionic. This is because the polar. When salt is added to oil, it will simply sink to the bottom and remain there. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. In short, no, salt does not dissolve in oil. Why does salt dissolve in water but not oil? Salt dissolves in water due to the polarity of water molecules, which allows them to attract and. Ionic compounds, like table salt (sodium chloride), dissolve easily in water. Water molecules move about continuously due to their kinetic. Water typically dissolves most ionic compounds and polar molecules. Salt dissolves in water because both substances have polar molecules, while oil.
from www.vectorstock.com
This is because the polar. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Salt dissolves in water because both substances have polar molecules, while oil. Water typically dissolves most ionic compounds and polar molecules. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. We will first examine the process that occurs when an ionic. Water molecules move about continuously due to their kinetic. When it comes to the simple question of whether salt will dissolve in oil, the answer is no. Nonpolar molecules such as those found in grease or oil do not dissolve in water. When salt is added to water, the polar water molecules surround the charged ions (sodium and chloride), pulling them away from each other and dispersing them evenly throughout the solution.
How does sodium chloride nacl dissolve in water Vector Image
Why Does Table Salt Dissolve In Water But Not Cooking Oil Water typically dissolves most ionic compounds and polar molecules. When salt is added to water, the polar water molecules surround the charged ions (sodium and chloride), pulling them away from each other and dispersing them evenly throughout the solution. Salt dissolves in water because both substances have polar molecules, while oil. Ionic compounds, like table salt (sodium chloride), dissolve easily in water. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Water typically dissolves most ionic compounds and polar molecules. Why does salt dissolve in water but not oil? Water molecules move about continuously due to their kinetic. When salt is added to oil, it will simply sink to the bottom and remain there. When it comes to the simple question of whether salt will dissolve in oil, the answer is no. We will first examine the process that occurs when an ionic. Why does salt dissolve in water but not in oil? In short, no, salt does not dissolve in oil. Nonpolar molecules such as those found in grease or oil do not dissolve in water. This is because the polar.
From www.wikihow.com
How to Dissolve Salt in Water 9 Steps (with Pictures) wikiHow Why Does Table Salt Dissolve In Water But Not Cooking Oil Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Water typically dissolves most ionic compounds and polar molecules. When salt is added to water, the polar water molecules surround the charged ions (sodium and chloride), pulling them away from each other and dispersing them evenly throughout the solution. Salt dissolves in water due to. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Why Does Table Salt Dissolve In Water But Not Cooking Oil When salt is added to water, the polar water molecules surround the charged ions (sodium and chloride), pulling them away from each other and dispersing them evenly throughout the solution. We will first examine the process that occurs when an ionic. Why does salt dissolve in water but not oil? This is because the polar. Why does salt dissolve in. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From www.bbc.co.uk
Which materials dissolve in water? BBC Bitesize Why Does Table Salt Dissolve In Water But Not Cooking Oil Water typically dissolves most ionic compounds and polar molecules. Water molecules move about continuously due to their kinetic. When salt is added to water, the polar water molecules surround the charged ions (sodium and chloride), pulling them away from each other and dispersing them evenly throughout the solution. When it comes to the simple question of whether salt will dissolve. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From ar.inspiredpencil.com
Solubility Examples Why Does Table Salt Dissolve In Water But Not Cooking Oil Salt dissolves in water because both substances have polar molecules, while oil. Salt dissolves in water due to the polarity of water molecules, which allows them to attract and. Water molecules move about continuously due to their kinetic. Why does salt dissolve in water but not in oil? Nonpolar molecules such as those found in grease or oil do not. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From www.wikihow.com
How to Dissolve Salt in Water 9 Steps (with Pictures) wikiHow Why Does Table Salt Dissolve In Water But Not Cooking Oil Salt dissolves in water because both substances have polar molecules, while oil. Water typically dissolves most ionic compounds and polar molecules. In short, no, salt does not dissolve in oil. When salt is added to water, the polar water molecules surround the charged ions (sodium and chloride), pulling them away from each other and dispersing them evenly throughout the solution.. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID Why Does Table Salt Dissolve In Water But Not Cooking Oil When salt is added to water, the polar water molecules surround the charged ions (sodium and chloride), pulling them away from each other and dispersing them evenly throughout the solution. Water typically dissolves most ionic compounds and polar molecules. We will first examine the process that occurs when an ionic. This is because the polar. We will first examine the. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From eduvik.in
Matter in Our Surroundings Class 9 Notes Science Chapter 1 Eduvik Why Does Table Salt Dissolve In Water But Not Cooking Oil Water molecules move about continuously due to their kinetic. Nonpolar molecules such as those found in grease or oil do not dissolve in water. Salt dissolves in water due to the polarity of water molecules, which allows them to attract and. We will first examine the process that occurs when an ionic. Water typically dissolves most ionic compounds and polar. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From www.aquriousmind.com
Why does salt dissolve in water? AQuriousMind Why Does Table Salt Dissolve In Water But Not Cooking Oil Nonpolar molecules such as those found in grease or oil do not dissolve in water. This is because the polar. When it comes to the simple question of whether salt will dissolve in oil, the answer is no. Ionic compounds, like table salt (sodium chloride), dissolve easily in water. We will first examine the process that occurs when an ionic. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From curiosityguide.org
How Does Salt Dissolve in Water? Curiosity Guide Why Does Table Salt Dissolve In Water But Not Cooking Oil When it comes to the simple question of whether salt will dissolve in oil, the answer is no. When salt is added to oil, it will simply sink to the bottom and remain there. We will first examine the process that occurs when an ionic. Why does salt dissolve in water but not in oil? Ionic compounds, like table salt. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From studylibstearine.z21.web.core.windows.net
How Does Water Dissolve Salt Why Does Table Salt Dissolve In Water But Not Cooking Oil Why does salt dissolve in water but not in oil? Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. In short, no, salt does not dissolve in oil. Why does salt dissolve in water but not oil? Ionic compounds, like table salt (sodium chloride), dissolve easily in water. Water molecules move about continuously due. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From www.slideserve.com
PPT Chemical Reactions Chapter 7 PowerPoint Presentation, free Why Does Table Salt Dissolve In Water But Not Cooking Oil When it comes to the simple question of whether salt will dissolve in oil, the answer is no. In short, no, salt does not dissolve in oil. Nonpolar molecules such as those found in grease or oil do not dissolve in water. Why does salt dissolve in water but not oil? Water molecules move about continuously due to their kinetic.. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From schematicfixtrysted.z22.web.core.windows.net
Diagram Of Salt Dissolving In Water Why Does Table Salt Dissolve In Water But Not Cooking Oil When salt is added to oil, it will simply sink to the bottom and remain there. Ionic compounds, like table salt (sodium chloride), dissolve easily in water. Salt dissolves in water due to the polarity of water molecules, which allows them to attract and. When salt is added to water, the polar water molecules surround the charged ions (sodium and. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From www.youtube.com
table salt dissolves in water YouTube Why Does Table Salt Dissolve In Water But Not Cooking Oil Why does salt dissolve in water but not oil? Salt dissolves in water because both substances have polar molecules, while oil. When salt is added to water, the polar water molecules surround the charged ions (sodium and chloride), pulling them away from each other and dispersing them evenly throughout the solution. We will first examine the process that occurs when. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From ar.inspiredpencil.com
Solubility Of Salt Why Does Table Salt Dissolve In Water But Not Cooking Oil Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. When salt is added to oil, it will simply sink to the bottom and remain there. Why does salt dissolve in water but not in oil? This is because the polar. Water typically dissolves most ionic compounds and polar molecules. We will first examine the. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From www.vectorstock.com
How does sodium chloride nacl dissolve in water Vector Image Why Does Table Salt Dissolve In Water But Not Cooking Oil Water typically dissolves most ionic compounds and polar molecules. Salt dissolves in water because both substances have polar molecules, while oil. When salt is added to oil, it will simply sink to the bottom and remain there. When it comes to the simple question of whether salt will dissolve in oil, the answer is no. We will first examine the. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From www.slideserve.com
PPT Chapter 7 Chemical Reactions PowerPoint Presentation, free Why Does Table Salt Dissolve In Water But Not Cooking Oil When it comes to the simple question of whether salt will dissolve in oil, the answer is no. Salt dissolves in water due to the polarity of water molecules, which allows them to attract and. Water molecules move about continuously due to their kinetic. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Water. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From www.wou.edu
CH150 Chapter 7 Solutions Chemistry Why Does Table Salt Dissolve In Water But Not Cooking Oil When salt is added to water, the polar water molecules surround the charged ions (sodium and chloride), pulling them away from each other and dispersing them evenly throughout the solution. Nonpolar molecules such as those found in grease or oil do not dissolve in water. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water.. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From thetruthfacts.com
Does Salt Dissolve In Oil A Beginner's Guide Why Does Table Salt Dissolve In Water But Not Cooking Oil Water molecules move about continuously due to their kinetic. Salt dissolves in water due to the polarity of water molecules, which allows them to attract and. When salt is added to water, the polar water molecules surround the charged ions (sodium and chloride), pulling them away from each other and dispersing them evenly throughout the solution. Ionic compounds, like table. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From www.youtube.com
Why oil does not dissolve in water Polarity of water YouTube Why Does Table Salt Dissolve In Water But Not Cooking Oil Water typically dissolves most ionic compounds and polar molecules. In short, no, salt does not dissolve in oil. Nonpolar molecules such as those found in grease or oil do not dissolve in water. Salt dissolves in water because both substances have polar molecules, while oil. When salt is added to oil, it will simply sink to the bottom and remain. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From www.youtube.com
table salt dissolves in water YouTube Why Does Table Salt Dissolve In Water But Not Cooking Oil Water typically dissolves most ionic compounds and polar molecules. When it comes to the simple question of whether salt will dissolve in oil, the answer is no. This is because the polar. Salt dissolves in water due to the polarity of water molecules, which allows them to attract and. Why does salt dissolve in water but not oil? Ionic compounds,. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From thetruthfacts.com
Does Salt Dissolve In Oil A Beginner's Guide Why Does Table Salt Dissolve In Water But Not Cooking Oil We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Ionic compounds, like table salt (sodium chloride), dissolve easily in water. Why does salt dissolve in water but not in oil? When salt is added to oil, it will simply sink to the bottom and remain there. Salt dissolves. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From powerupcook.com
How Does Salt Dissolve In Water Power Up Cook Why Does Table Salt Dissolve In Water But Not Cooking Oil Water molecules move about continuously due to their kinetic. Ionic compounds, like table salt (sodium chloride), dissolve easily in water. When it comes to the simple question of whether salt will dissolve in oil, the answer is no. Why does salt dissolve in water but not in oil? Nonpolar molecules such as those found in grease or oil do not. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID762021 Why Does Table Salt Dissolve In Water But Not Cooking Oil In short, no, salt does not dissolve in oil. Ionic compounds, like table salt (sodium chloride), dissolve easily in water. We will first examine the process that occurs when an ionic. Salt dissolves in water due to the polarity of water molecules, which allows them to attract and. Nonpolar molecules such as those found in grease or oil do not. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From mungfali.com
Dissolving Salt In Water Diagram Why Does Table Salt Dissolve In Water But Not Cooking Oil We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. When it comes to the simple question of whether salt will dissolve in oil, the answer is no. Why does salt dissolve in water but not oil? Nonpolar molecules such as those found in grease or oil do not. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From sciencenotes.org
Solubility Rules Chart and Memorization Tips Why Does Table Salt Dissolve In Water But Not Cooking Oil Why does salt dissolve in water but not in oil? Water typically dissolves most ionic compounds and polar molecules. When it comes to the simple question of whether salt will dissolve in oil, the answer is no. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. In short, no, salt does not dissolve in. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From www.wikidoc.org
Solution wikidoc Why Does Table Salt Dissolve In Water But Not Cooking Oil We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Why does salt dissolve in water but not oil? Salt dissolves in water because both substances have polar molecules, while oil. Nonpolar molecules such as those found in grease or oil do not dissolve in water. Why does salt. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From www.youtube.com
Why Salt (NaCl) Does Not Dissolve in Oil Salt and Oil Experiment Is Why Does Table Salt Dissolve In Water But Not Cooking Oil This is because the polar. Water typically dissolves most ionic compounds and polar molecules. Why does salt dissolve in water but not in oil? Water molecules move about continuously due to their kinetic. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. When salt is added to oil, it will simply sink to the. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From handletheheat.com
Kosher Salt vs. Sea Salt vs. Table Salt Handle the Heat Why Does Table Salt Dissolve In Water But Not Cooking Oil Why does salt dissolve in water but not in oil? When it comes to the simple question of whether salt will dissolve in oil, the answer is no. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Water typically dissolves most ionic compounds and polar molecules. In short,. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From www.slideserve.com
PPT Chapter 2 Chemistry PowerPoint Presentation, free Why Does Table Salt Dissolve In Water But Not Cooking Oil Ionic compounds, like table salt (sodium chloride), dissolve easily in water. Salt dissolves in water due to the polarity of water molecules, which allows them to attract and. We will first examine the process that occurs when an ionic. Why does salt dissolve in water but not oil? When salt is added to oil, it will simply sink to the. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From www.baristahustle.com
TWC 0.02 How Does Water Dissolve Mineral Salts? Barista Hustle Why Does Table Salt Dissolve In Water But Not Cooking Oil Salt dissolves in water because both substances have polar molecules, while oil. Water typically dissolves most ionic compounds and polar molecules. Why does salt dissolve in water but not oil? This is because the polar. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Nonpolar molecules, such as. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From shaunmwilliams.com
Chapter 11 Presentation Why Does Table Salt Dissolve In Water But Not Cooking Oil Why does salt dissolve in water but not in oil? When salt is added to oil, it will simply sink to the bottom and remain there. Ionic compounds, like table salt (sodium chloride), dissolve easily in water. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Salt dissolves in water due to the polarity. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From heaventaste.org
Discovering Compatibility Does Salt Dissolve in Oil? Heaven Taste Why Does Table Salt Dissolve In Water But Not Cooking Oil Nonpolar molecules such as those found in grease or oil do not dissolve in water. Water typically dissolves most ionic compounds and polar molecules. Ionic compounds, like table salt (sodium chloride), dissolve easily in water. Why does salt dissolve in water but not in oil? When salt is added to oil, it will simply sink to the bottom and remain. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From thetruthfacts.com
Does Salt Dissolve In Oil A Beginner's Guide Why Does Table Salt Dissolve In Water But Not Cooking Oil We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Ionic compounds, like table salt (sodium chloride), dissolve easily in water. Nonpolar molecules such as those found in grease or oil do not dissolve. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From www.youtube.com
How Salt Dissolves in Water? YouTube Why Does Table Salt Dissolve In Water But Not Cooking Oil Why does salt dissolve in water but not oil? Salt dissolves in water due to the polarity of water molecules, which allows them to attract and. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Nonpolar molecules such as those found in grease or oil do not dissolve in water. Salt dissolves in water. Why Does Table Salt Dissolve In Water But Not Cooking Oil.
From eichyler.us.to
Why Does Salt Dissolve in Water But Not Oil? LEAFtv Why Does Table Salt Dissolve In Water But Not Cooking Oil When salt is added to oil, it will simply sink to the bottom and remain there. Why does salt dissolve in water but not oil? When it comes to the simple question of whether salt will dissolve in oil, the answer is no. Salt dissolves in water because both substances have polar molecules, while oil. We will first examine the. Why Does Table Salt Dissolve In Water But Not Cooking Oil.