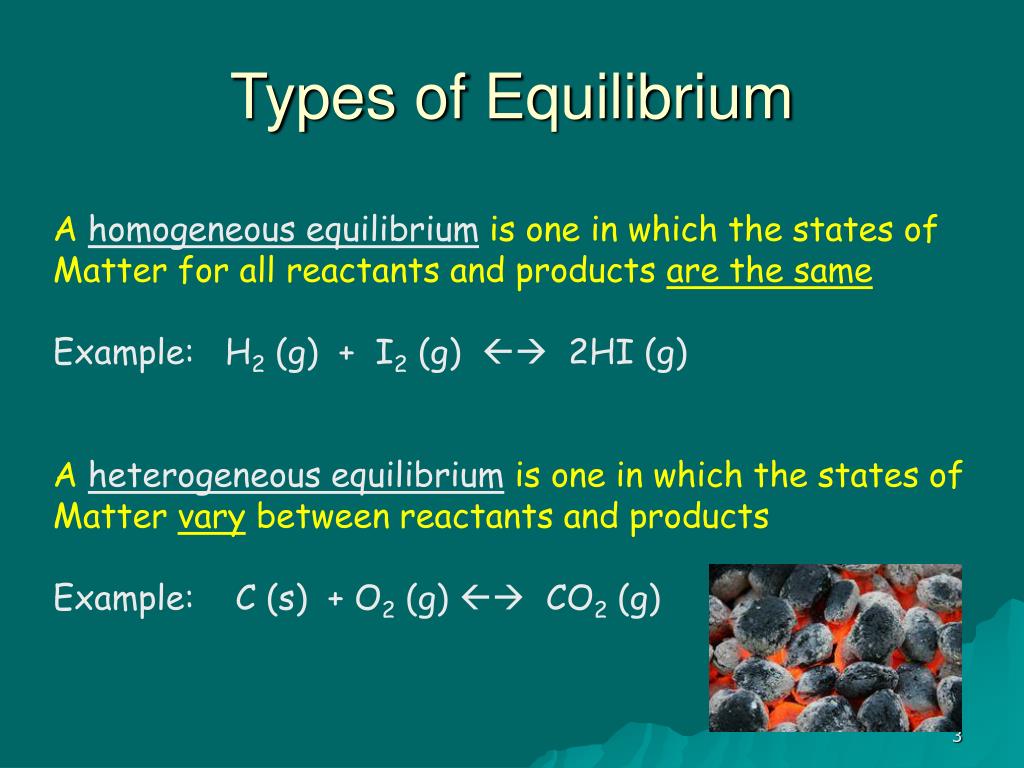
Solid-Liquid Equilibrium Chemistry . equilibrium can be established for both physical processes and chemical reactions. The reaction may be fast or slow. The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture of the two solids has the lowest melting point. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. — consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: — a phase diagram for two immiscible solids and the liquid phase (which is miscible in all proportions) is shown in figure \(\pageindex{1}\).
from www.slideserve.com
The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture of the two solids has the lowest melting point. equilibrium can be established for both physical processes and chemical reactions. — consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The reaction may be fast or slow. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. — a phase diagram for two immiscible solids and the liquid phase (which is miscible in all proportions) is shown in figure \(\pageindex{1}\).
PPT Chemical Equilibrium PowerPoint Presentation, free download ID
Solid-Liquid Equilibrium Chemistry equilibrium can be established for both physical processes and chemical reactions. — consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: — a phase diagram for two immiscible solids and the liquid phase (which is miscible in all proportions) is shown in figure \(\pageindex{1}\). The reaction may be fast or slow. The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture of the two solids has the lowest melting point. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. equilibrium can be established for both physical processes and chemical reactions.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation ID3889861 Solid-Liquid Equilibrium Chemistry The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture of the two solids has the lowest melting point. The reaction may be fast or slow. — a phase diagram for two immiscible solids and the liquid phase (which is miscible in all proportions) is shown in figure \(\pageindex{1}\). equilibrium. Solid-Liquid Equilibrium Chemistry.
From schematichettum57.z21.web.core.windows.net
How To Read Phase Diagrams Chemistry Solid-Liquid Equilibrium Chemistry — consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: equilibrium can be established for both physical processes and chemical reactions. The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture of the two solids has the lowest melting. Solid-Liquid Equilibrium Chemistry.
From www.slideshare.net
Chemistry Equilibrium Solid-Liquid Equilibrium Chemistry The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture of the two solids has the lowest melting point. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. equilibrium can be established for both physical processes and chemical reactions. — consider an equilibrium between a crystalline. Solid-Liquid Equilibrium Chemistry.
From www.shutterstock.com
Solid Liquid Equilibrium Over 101 RoyaltyFree Licensable Stock Solid-Liquid Equilibrium Chemistry — consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. equilibrium can be established for both physical processes and chemical reactions. The point labeled “e 2 ” is the eutectic point , meaning the. Solid-Liquid Equilibrium Chemistry.
From www.researchgate.net
Phase diagram and the phase equilibrium line 1 solid and liquid Solid-Liquid Equilibrium Chemistry The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. — a phase diagram for two immiscible solids and the liquid phase (which is miscible in all proportions) is shown in figure \(\pageindex{1}\). equilibrium can be established for both physical processes and chemical reactions. The reaction may be fast or slow. The point labeled “e. Solid-Liquid Equilibrium Chemistry.
From www.youtube.com
SolidLiquid Chemical Equilibrium YouTube Solid-Liquid Equilibrium Chemistry — consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. The reaction may be fast or slow. — a phase diagram for two immiscible solids and the liquid phase (which is miscible in all. Solid-Liquid Equilibrium Chemistry.
From www.numerade.com
SOLVEDWrite an equilibrium expression for each chemical equation Solid-Liquid Equilibrium Chemistry The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture of the two solids has the lowest melting point. equilibrium can be established for both physical processes and chemical reactions. The reaction may be fast or slow. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. . Solid-Liquid Equilibrium Chemistry.
From www.snexplores.org
Explainer What are the different states of matter? Solid-Liquid Equilibrium Chemistry The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture of the two solids has the lowest melting point. — a phase diagram for two immiscible solids and the liquid phase (which is miscible in all proportions) is shown in figure \(\pageindex{1}\). — consider an equilibrium between a crystalline salt. Solid-Liquid Equilibrium Chemistry.
From www.researchgate.net
Phase diagram of solid liquid equilibrium in the system H 2 O FeSO Solid-Liquid Equilibrium Chemistry The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture of the two solids has the lowest melting point. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. — a phase diagram for two immiscible solids and the liquid phase (which is miscible in all proportions) is. Solid-Liquid Equilibrium Chemistry.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM PowerPoint Presentation, free download ID Solid-Liquid Equilibrium Chemistry The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. equilibrium can be established for both physical processes and chemical reactions. — consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The reaction may be fast or slow. The point labeled “e 2 ”. Solid-Liquid Equilibrium Chemistry.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Solid-Liquid Equilibrium Chemistry The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. — consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: — a phase diagram for two immiscible solids and the liquid phase (which is miscible in all proportions) is shown in figure \(\pageindex{1}\). The. Solid-Liquid Equilibrium Chemistry.
From www.slideserve.com
PPT Unit 2 Liquids and solids, solubility, equilibrium PowerPoint Solid-Liquid Equilibrium Chemistry — consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: equilibrium can be established for both physical processes and chemical reactions. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. The reaction may be fast or slow. — a phase diagram for. Solid-Liquid Equilibrium Chemistry.
From chem.libretexts.org
12.5 SolidLiquid Equilibria Chemistry LibreTexts Solid-Liquid Equilibrium Chemistry The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. — consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture of the two solids has the lowest. Solid-Liquid Equilibrium Chemistry.
From chem.libretexts.org
12.5 SolidLiquid Equilibria Chemistry LibreTexts Solid-Liquid Equilibrium Chemistry equilibrium can be established for both physical processes and chemical reactions. — consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. The point labeled “e 2 ” is the eutectic point , meaning the. Solid-Liquid Equilibrium Chemistry.
From www.slideserve.com
PPT Types of Solids PowerPoint Presentation, free download ID4500701 Solid-Liquid Equilibrium Chemistry The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture of the two solids has the lowest melting point. equilibrium can be established for both physical processes and chemical reactions. — a phase diagram for two immiscible solids and the liquid phase (which is miscible in all proportions) is shown. Solid-Liquid Equilibrium Chemistry.
From www.scribd.com
Solid Liquid Equilibrium Phase (Matter) Phase Diagram Solid-Liquid Equilibrium Chemistry The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture of the two solids has the lowest melting point. The reaction may be fast or slow. — a phase diagram for two immiscible solids and the liquid phase (which is miscible in all proportions) is shown in figure \(\pageindex{1}\). —. Solid-Liquid Equilibrium Chemistry.
From sciencetallis.weebly.com
3. Particle Model of Matter THOMAS TALLIS SCIENCE Solid-Liquid Equilibrium Chemistry The reaction may be fast or slow. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture of the two solids has the lowest melting point. equilibrium can be established for both physical processes and chemical reactions. . Solid-Liquid Equilibrium Chemistry.
From chem.libretexts.org
12.5 SolidLiquid Equilibria Chemistry LibreTexts Solid-Liquid Equilibrium Chemistry equilibrium can be established for both physical processes and chemical reactions. — consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The reaction may be fast or slow. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. — a phase diagram for. Solid-Liquid Equilibrium Chemistry.
From www.mdpi.com
Molecules Free FullText Modeling of SolidLiquid Equilibria in Solid-Liquid Equilibrium Chemistry equilibrium can be established for both physical processes and chemical reactions. The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture of the two solids has the lowest melting point. The reaction may be fast or slow. — consider an equilibrium between a crystalline salt (or other kind of ionic. Solid-Liquid Equilibrium Chemistry.
From studylib.net
Solid Liquid Vapor equilibria phase diagrams Solid-Liquid Equilibrium Chemistry The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture of the two solids has the lowest melting point. equilibrium can be established for both physical processes and chemical reactions. The reaction may be fast or slow. — consider an equilibrium between a crystalline salt (or other kind of ionic. Solid-Liquid Equilibrium Chemistry.
From www.researchgate.net
Experimental solidliquid equilibrium (SLE) phase diagrams ( ) of the Solid-Liquid Equilibrium Chemistry equilibrium can be established for both physical processes and chemical reactions. — a phase diagram for two immiscible solids and the liquid phase (which is miscible in all proportions) is shown in figure \(\pageindex{1}\). — consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The thermodynamics. Solid-Liquid Equilibrium Chemistry.
From learncheme.com
ssleequilibrium LearnChemE Solid-Liquid Equilibrium Chemistry The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. The reaction may be fast or slow. The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture of the two solids has the lowest melting point. — a phase diagram for two immiscible solids and the liquid phase. Solid-Liquid Equilibrium Chemistry.
From byjus.com
Equilibrium Involving Dissolution Of Solid Gas In Liquid Henry's Law Solid-Liquid Equilibrium Chemistry The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture of the two solids has the lowest melting point. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. — a phase diagram for two immiscible solids and the liquid phase (which is miscible in all proportions) is. Solid-Liquid Equilibrium Chemistry.
From www.numerade.com
SOLVEDIn a final equilibrium expression solids and liquid have value Solid-Liquid Equilibrium Chemistry equilibrium can be established for both physical processes and chemical reactions. The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture of the two solids has the lowest melting point. The reaction may be fast or slow. — consider an equilibrium between a crystalline salt (or other kind of ionic. Solid-Liquid Equilibrium Chemistry.
From learncheme.com
solidsolidliquidphasediagramsconceptestandexampleproblem Solid-Liquid Equilibrium Chemistry — a phase diagram for two immiscible solids and the liquid phase (which is miscible in all proportions) is shown in figure \(\pageindex{1}\). The reaction may be fast or slow. equilibrium can be established for both physical processes and chemical reactions. The point labeled “e 2 ” is the eutectic point , meaning the composition for which the. Solid-Liquid Equilibrium Chemistry.
From www.slideserve.com
PPT The Concept of Equilibrium PowerPoint Presentation, free download Solid-Liquid Equilibrium Chemistry The reaction may be fast or slow. — consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. — a phase diagram for two immiscible solids and the liquid phase (which is miscible in all. Solid-Liquid Equilibrium Chemistry.
From www.youtube.com
SolidLiquid Equilibrium YouTube Solid-Liquid Equilibrium Chemistry The reaction may be fast or slow. — a phase diagram for two immiscible solids and the liquid phase (which is miscible in all proportions) is shown in figure \(\pageindex{1}\). The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture of the two solids has the lowest melting point. equilibrium. Solid-Liquid Equilibrium Chemistry.
From schematicviciosinfin17.z22.web.core.windows.net
How To Make A Phase Diagram Solid-Liquid Equilibrium Chemistry — consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The reaction may be fast or slow. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture. Solid-Liquid Equilibrium Chemistry.
From chem.libretexts.org
5.6 Phase Diagrams Chemistry LibreTexts Solid-Liquid Equilibrium Chemistry equilibrium can be established for both physical processes and chemical reactions. — a phase diagram for two immiscible solids and the liquid phase (which is miscible in all proportions) is shown in figure \(\pageindex{1}\). The reaction may be fast or slow. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. — consider an. Solid-Liquid Equilibrium Chemistry.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Solid-Liquid Equilibrium Chemistry — consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. — a phase diagram for two immiscible solids and the liquid phase (which is miscible in all proportions) is shown in figure \(\pageindex{1}\). The. Solid-Liquid Equilibrium Chemistry.
From courses.lumenlearning.com
Phase Diagrams Chemistry for Majors Solid-Liquid Equilibrium Chemistry The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. — a phase diagram for two immiscible solids and the liquid phase (which is miscible in all proportions) is shown in figure \(\pageindex{1}\). The reaction may be fast or slow. The point labeled “e 2 ” is the eutectic point , meaning the composition for which. Solid-Liquid Equilibrium Chemistry.
From chem.libretexts.org
12.5 SolidLiquid Equilibria Chemistry LibreTexts Solid-Liquid Equilibrium Chemistry equilibrium can be established for both physical processes and chemical reactions. The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture of the two solids has the lowest melting point. — a phase diagram for two immiscible solids and the liquid phase (which is miscible in all proportions) is shown. Solid-Liquid Equilibrium Chemistry.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Solid-Liquid Equilibrium Chemistry The reaction may be fast or slow. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. equilibrium can be established for both physical processes and chemical reactions. The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture of the two solids has the lowest melting point. . Solid-Liquid Equilibrium Chemistry.
From www.slideserve.com
PPT Unit 2 Liquids & solids, solubility, equilibrium PowerPoint Solid-Liquid Equilibrium Chemistry The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. The reaction may be fast or slow. — a phase diagram for two immiscible solids and the liquid phase (which is miscible in all proportions) is shown in figure \(\pageindex{1}\). — consider an equilibrium between a crystalline salt (or other kind of ionic solid) and. Solid-Liquid Equilibrium Chemistry.
From www.len.com.ng
Chemical Equilibrium Dynamic Equilibrium in Chemistry Solid-Liquid Equilibrium Chemistry The point labeled “e 2 ” is the eutectic point , meaning the composition for which the mixture of the two solids has the lowest melting point. The reaction may be fast or slow. equilibrium can be established for both physical processes and chemical reactions. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline. . Solid-Liquid Equilibrium Chemistry.