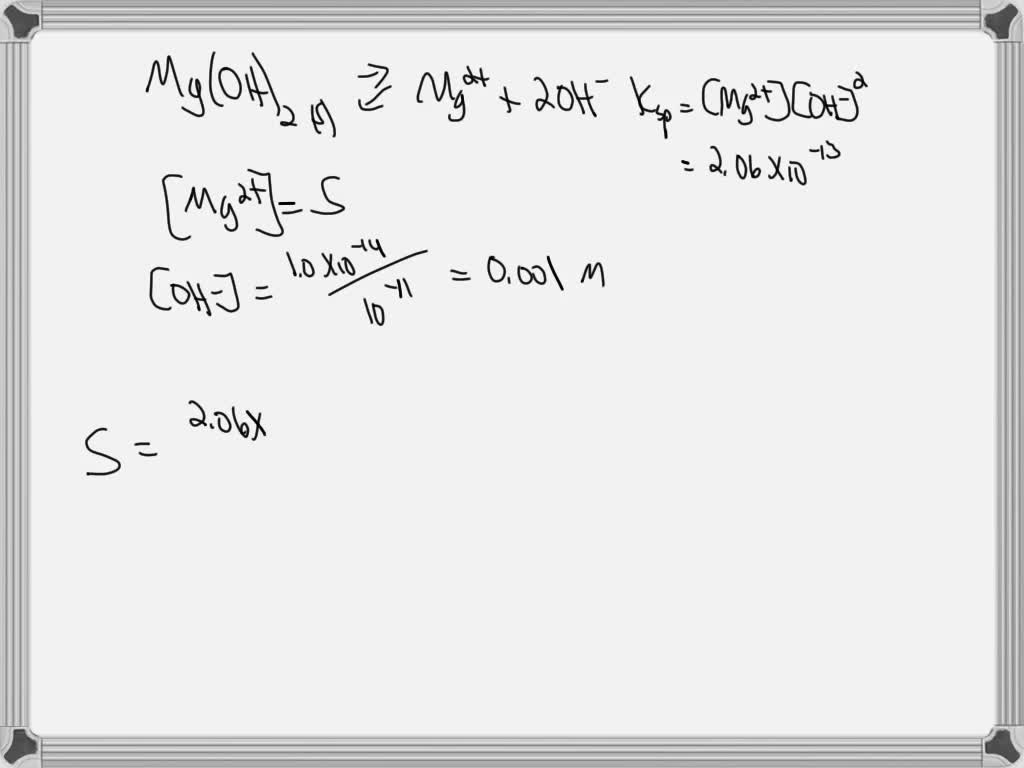Magnesium Hydroxide Ksp . 3) use the ph to get. 2) the k sp expression: Mg (oh) 2 ⇌ mg 2+ + 2oh¯. We hope they will prove usefull to you. 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. K sp = [mg 2+] [oh¯] 2. 176 rows — the table below gives calculated values of k sp for common ionic substances at 25°c (77°f). below are the values of the ksp product constant for the most common salts. — solubility product constants (ksq k s q) are given to those solutes, and these constants can be used to find the. For every mole of magnesium.
from www.numerade.com
We hope they will prove usefull to you. 2) the k sp expression: 176 rows — the table below gives calculated values of k sp for common ionic substances at 25°c (77°f). For every mole of magnesium. 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. K sp = [mg 2+] [oh¯] 2. below are the values of the ksp product constant for the most common salts. — solubility product constants (ksq k s q) are given to those solutes, and these constants can be used to find the. Mg (oh) 2 ⇌ mg 2+ + 2oh¯. 3) use the ph to get.
SOLVED Calculate the solubility (in grams per 10 mL of solution) of
Magnesium Hydroxide Ksp Mg (oh) 2 ⇌ mg 2+ + 2oh¯. 3) use the ph to get. 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. K sp = [mg 2+] [oh¯] 2. 176 rows — the table below gives calculated values of k sp for common ionic substances at 25°c (77°f). We hope they will prove usefull to you. For every mole of magnesium. 2) the k sp expression: — solubility product constants (ksq k s q) are given to those solutes, and these constants can be used to find the. Mg (oh) 2 ⇌ mg 2+ + 2oh¯. below are the values of the ksp product constant for the most common salts.
From www.chegg.com
Solved Determine the solubility of magnesium hydroxide Magnesium Hydroxide Ksp 2) the k sp expression: For every mole of magnesium. We hope they will prove usefull to you. — solubility product constants (ksq k s q) are given to those solutes, and these constants can be used to find the. 176 rows — the table below gives calculated values of k sp for common ionic substances at 25°c. Magnesium Hydroxide Ksp.
From www.researchgate.net
(a) Molecular structure diagram of magnesium hydroxide (b) Crystal Magnesium Hydroxide Ksp 3) use the ph to get. We hope they will prove usefull to you. below are the values of the ksp product constant for the most common salts. Mg (oh) 2 ⇌ mg 2+ + 2oh¯. — solubility product constants (ksq k s q) are given to those solutes, and these constants can be used to find the.. Magnesium Hydroxide Ksp.
From www.indiamart.com
Technical Grade Magnesium Hydroxide IP BP USP for Laboratory, Rs 86 /kg Magnesium Hydroxide Ksp 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. below are the values of the ksp product constant for the most common salts. 2) the k sp expression: 176 rows — the table below gives calculated values of k sp for common ionic substances at 25°c (77°f). For. Magnesium Hydroxide Ksp.
From www.numerade.com
SOLVED Try These On Your Own At 25°C, the pH of a saturated solution Magnesium Hydroxide Ksp 2) the k sp expression: 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. Mg (oh) 2 ⇌ mg 2+ + 2oh¯. 176 rows — the table below gives calculated values of k sp for common ionic substances at 25°c (77°f). below are the values of the ksp. Magnesium Hydroxide Ksp.
From www.numerade.com
SOLVED A. Calculate the solubility (in g/L) of magnesium fluoride (Ksp Magnesium Hydroxide Ksp For every mole of magnesium. below are the values of the ksp product constant for the most common salts. 3) use the ph to get. 2) the k sp expression: 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. Mg (oh) 2 ⇌ mg 2+ + 2oh¯. K sp. Magnesium Hydroxide Ksp.
From byjus.com
Solubility product of magnesium Hydroxide is 4 10 12 . The number of Magnesium Hydroxide Ksp 2) the k sp expression: We hope they will prove usefull to you. 3) use the ph to get. below are the values of the ksp product constant for the most common salts. 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. For every mole of magnesium. —. Magnesium Hydroxide Ksp.
From testbook.com
Magnesium Hydroxide Definition, Symbol, Examples, Structure Magnesium Hydroxide Ksp 3) use the ph to get. For every mole of magnesium. below are the values of the ksp product constant for the most common salts. 176 rows — the table below gives calculated values of k sp for common ionic substances at 25°c (77°f). 2) the k sp expression: Mg (oh) 2 ⇌ mg 2+ + 2oh¯. . Magnesium Hydroxide Ksp.
From www.numerade.com
SOLVED 23. The Kyof magnesium hydroxide is 1.2xl0" Find the Magnesium Hydroxide Ksp 2) the k sp expression: 3) use the ph to get. Mg (oh) 2 ⇌ mg 2+ + 2oh¯. 176 rows — the table below gives calculated values of k sp for common ionic substances at 25°c (77°f). For every mole of magnesium. — solubility product constants (ksq k s q) are given to those solutes, and these. Magnesium Hydroxide Ksp.
From www.chegg.com
Solved PostLaboratory Question The Ksp of magnesium Magnesium Hydroxide Ksp For every mole of magnesium. We hope they will prove usefull to you. 3) use the ph to get. K sp = [mg 2+] [oh¯] 2. Mg (oh) 2 ⇌ mg 2+ + 2oh¯. below are the values of the ksp product constant for the most common salts. — solubility product constants (ksq k s q) are given. Magnesium Hydroxide Ksp.
From www.chegg.com
Solved a 10. The Ksp of magnesium hydroxide is 1.2 x 1011. Magnesium Hydroxide Ksp — solubility product constants (ksq k s q) are given to those solutes, and these constants can be used to find the. 3) use the ph to get. 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. We hope they will prove usefull to you. below are the. Magnesium Hydroxide Ksp.
From www.chegg.com
3. The reaction between magnesium hydroxide and Magnesium Hydroxide Ksp — solubility product constants (ksq k s q) are given to those solutes, and these constants can be used to find the. For every mole of magnesium. 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. Mg (oh) 2 ⇌ mg 2+ + 2oh¯. 2) the k sp expression:. Magnesium Hydroxide Ksp.
From www.chegg.com
Solved The Molar Solubility Of Magnesium Hydroxide Is 1.1... Magnesium Hydroxide Ksp 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. 176 rows — the table below gives calculated values of k sp for common ionic substances at 25°c (77°f). Mg (oh) 2 ⇌ mg 2+ + 2oh¯. below are the values of the ksp product constant for the most. Magnesium Hydroxide Ksp.
From www.chegg.com
Solved Lab 10−Ksp of Magnesium Hydroxide In this lab we will Magnesium Hydroxide Ksp 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. For every mole of magnesium. We hope they will prove usefull to you. 2) the k sp expression: Mg (oh) 2 ⇌ mg 2+ + 2oh¯. 3) use the ph to get. K sp = [mg 2+] [oh¯] 2. below. Magnesium Hydroxide Ksp.
From www.numerade.com
SOLVEDThe solubility of Mg(OH)2 in water is approximately 9 mg / L at Magnesium Hydroxide Ksp 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. Mg (oh) 2 ⇌ mg 2+ + 2oh¯. 2) the k sp expression: 3) use the ph to get. 176 rows — the table below gives calculated values of k sp for common ionic substances at 25°c (77°f). —. Magnesium Hydroxide Ksp.
From www.numerade.com
SOLVED Given that the Ksp for Magnesium Hydroxide is 1.2 x 1011 Magnesium Hydroxide Ksp below are the values of the ksp product constant for the most common salts. 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. Mg (oh) 2 ⇌ mg 2+ + 2oh¯. — solubility product constants (ksq k s q) are given to those solutes, and these constants can. Magnesium Hydroxide Ksp.
From infinitylearn.com
Magnesium hydroxide Formula Infinity Learn Magnesium Hydroxide Ksp Mg (oh) 2 ⇌ mg 2+ + 2oh¯. We hope they will prove usefull to you. — solubility product constants (ksq k s q) are given to those solutes, and these constants can be used to find the. 176 rows — the table below gives calculated values of k sp for common ionic substances at 25°c (77°f). K. Magnesium Hydroxide Ksp.
From www.numerade.com
SOLVED Magnesium hydroxide is considered an insoluble compound Magnesium Hydroxide Ksp — solubility product constants (ksq k s q) are given to those solutes, and these constants can be used to find the. 3) use the ph to get. Mg (oh) 2 ⇌ mg 2+ + 2oh¯. 176 rows — the table below gives calculated values of k sp for common ionic substances at 25°c (77°f). 2) the k. Magnesium Hydroxide Ksp.
From www.numerade.com
SOLVED The pH of a saturated soloution of magnesium hydroxide, Mg(OH)2 Magnesium Hydroxide Ksp Mg (oh) 2 ⇌ mg 2+ + 2oh¯. For every mole of magnesium. 2) the k sp expression: 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. We hope they will prove usefull to you. below are the values of the ksp product constant for the most common salts.. Magnesium Hydroxide Ksp.
From www.numerade.com
SOLVED Determine the Ksp for magnesium hydroxide (Mg(OH)2) where the Magnesium Hydroxide Ksp 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. K sp = [mg 2+] [oh¯] 2. 3) use the ph to get. 176 rows — the table below gives calculated values of k sp for common ionic substances at 25°c (77°f). We hope they will prove usefull to you.. Magnesium Hydroxide Ksp.
From www.chegg.com
Solved Lab 10−Ksp of Magnesium Hydroxide In this lab we will Magnesium Hydroxide Ksp below are the values of the ksp product constant for the most common salts. 3) use the ph to get. We hope they will prove usefull to you. 176 rows — the table below gives calculated values of k sp for common ionic substances at 25°c (77°f). K sp = [mg 2+] [oh¯] 2. For every mole of. Magnesium Hydroxide Ksp.
From www.numerade.com
SOLVED Question 5 What is the equilibrium constant expression for the Magnesium Hydroxide Ksp 2) the k sp expression: For every mole of magnesium. K sp = [mg 2+] [oh¯] 2. 3) use the ph to get. below are the values of the ksp product constant for the most common salts. — solubility product constants (ksq k s q) are given to those solutes, and these constants can be used to find. Magnesium Hydroxide Ksp.
From www.numerade.com
SOLVED Text Magnesium hydroxide, Mg(OH)2, found in milk of magnesia Magnesium Hydroxide Ksp We hope they will prove usefull to you. — solubility product constants (ksq k s q) are given to those solutes, and these constants can be used to find the. 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. below are the values of the ksp product constant. Magnesium Hydroxide Ksp.
From www.numerade.com
SOLVED When magnesium hydroxide is dissolved in distilled water to Magnesium Hydroxide Ksp Mg (oh) 2 ⇌ mg 2+ + 2oh¯. 176 rows — the table below gives calculated values of k sp for common ionic substances at 25°c (77°f). 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. 2) the k sp expression: For every mole of magnesium. K sp =. Magnesium Hydroxide Ksp.
From www.numerade.com
SOLVED Complete the solubility product equation, the Kc and the Ksp Magnesium Hydroxide Ksp For every mole of magnesium. 176 rows — the table below gives calculated values of k sp for common ionic substances at 25°c (77°f). below are the values of the ksp product constant for the most common salts. We hope they will prove usefull to you. — solubility product constants (ksq k s q) are given to. Magnesium Hydroxide Ksp.
From www.numerade.com
SOLVED The solubility of Mg(OH)2 is measured and found to be 8.89×103 Magnesium Hydroxide Ksp We hope they will prove usefull to you. K sp = [mg 2+] [oh¯] 2. Mg (oh) 2 ⇌ mg 2+ + 2oh¯. For every mole of magnesium. below are the values of the ksp product constant for the most common salts. 3) use the ph to get. 176 rows — solubility product constant (ksp) (or the solubility. Magnesium Hydroxide Ksp.
From www.chegg.com
Solved (6 Pts) The Ksp of magnesium hydroxide, Mg(OH)2, is Magnesium Hydroxide Ksp 176 rows — the table below gives calculated values of k sp for common ionic substances at 25°c (77°f). We hope they will prove usefull to you. — solubility product constants (ksq k s q) are given to those solutes, and these constants can be used to find the. 176 rows — solubility product constant (ksp) (or. Magnesium Hydroxide Ksp.
From www.numerade.com
SOLVED should the illustrations The be altered t0 reflect this? Ksp Magnesium Hydroxide Ksp For every mole of magnesium. 2) the k sp expression: 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. below are the values of the ksp product constant for the most common salts. — solubility product constants (ksq k s q) are given to those solutes, and these. Magnesium Hydroxide Ksp.
From www.chegg.com
Solved Lab 10 Ksp of Magnesium Hydroxide In this lab we Magnesium Hydroxide Ksp 3) use the ph to get. 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. 2) the k sp expression: For every mole of magnesium. below are the values of the ksp product constant for the most common salts. 176 rows — the table below gives calculated values. Magnesium Hydroxide Ksp.
From www.chegg.com
Solved Magnesium HydroxideKsp Creafive Magnesium Hydroxide Ksp — solubility product constants (ksq k s q) are given to those solutes, and these constants can be used to find the. We hope they will prove usefull to you. For every mole of magnesium. Mg (oh) 2 ⇌ mg 2+ + 2oh¯. K sp = [mg 2+] [oh¯] 2. below are the values of the ksp product. Magnesium Hydroxide Ksp.
From www.numerade.com
SOLVEDFor the dissolution of magnesium hydroxide in water (a) Write a Magnesium Hydroxide Ksp 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. 176 rows — the table below gives calculated values of k sp for common ionic substances at 25°c (77°f). — solubility product constants (ksq k s q) are given to those solutes, and these constants can be used to. Magnesium Hydroxide Ksp.
From askfilo.com
The pH of aqueous solution of magnesium hydroxide is 9.0. If Ksp of magn.. Magnesium Hydroxide Ksp 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. 176 rows — the table below gives calculated values of k sp for common ionic substances at 25°c (77°f). For every mole of magnesium. We hope they will prove usefull to you. 3) use the ph to get. K sp. Magnesium Hydroxide Ksp.
From www.chegg.com
Solved Complete the solubility product equation, the Kc and Magnesium Hydroxide Ksp Mg (oh) 2 ⇌ mg 2+ + 2oh¯. K sp = [mg 2+] [oh¯] 2. below are the values of the ksp product constant for the most common salts. 176 rows — the table below gives calculated values of k sp for common ionic substances at 25°c (77°f). For every mole of magnesium. We hope they will prove. Magnesium Hydroxide Ksp.
From www.numerade.com
SOLVED Calculate the solubility (in grams per 10 mL of solution) of Magnesium Hydroxide Ksp K sp = [mg 2+] [oh¯] 2. 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. We hope they will prove usefull to you. 3) use the ph to get. — solubility product constants (ksq k s q) are given to those solutes, and these constants can be used. Magnesium Hydroxide Ksp.
From www.chegg.com
Solved 6. The Ksp of magnesium hydroxide is 5.6×10−12. Find Magnesium Hydroxide Ksp K sp = [mg 2+] [oh¯] 2. 2) the k sp expression: 3) use the ph to get. Mg (oh) 2 ⇌ mg 2+ + 2oh¯. — solubility product constants (ksq k s q) are given to those solutes, and these constants can be used to find the. 176 rows — the table below gives calculated values of. Magnesium Hydroxide Ksp.
From www.chegg.com
Solved 3. Magnesium hydroxide has a Ksp = 6.0x 1012 at 25° Magnesium Hydroxide Ksp For every mole of magnesium. 176 rows — the table below gives calculated values of k sp for common ionic substances at 25°c (77°f). 3) use the ph to get. 176 rows — solubility product constant (ksp) (or the solubility product) is the product of the molar concentrations. below are the values of the ksp product constant. Magnesium Hydroxide Ksp.