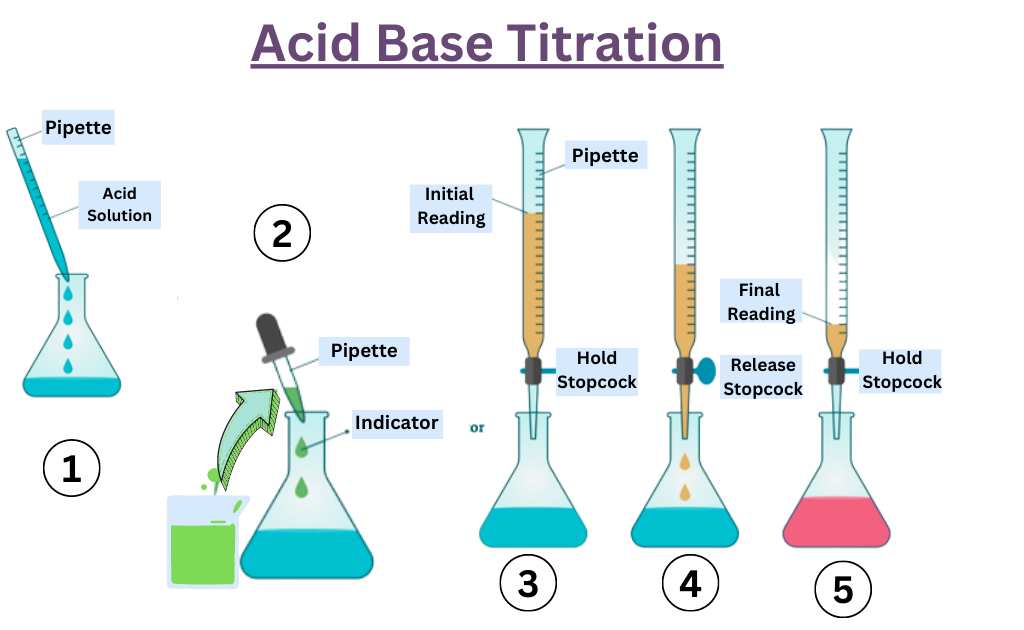
Acid Base Titration Lab Procedure . Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. Follow the procedure for preparing solutions, standardizing naoh, and titrating khp with naoh. See examples, definitions, and tips for using indicators and stoichiometry. For part a you will consider three types of reactions: A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The concentration of a solution can be determined by knowing the acid and base dissociation constant. Learn how to determine the concentration of unknown acids or bases by neutralizing them with a known solution.
from themasterchemistry.com
The analyte (titrand) is the solution with an unknown molarity. See examples, definitions, and tips for using indicators and stoichiometry. For part a you will consider three types of reactions: Follow the procedure for preparing solutions, standardizing naoh, and titrating khp with naoh. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The reagent (titrant) is the solution with a known molarity that will react with the analyte. Learn how to determine the concentration of unknown acids or bases by neutralizing them with a known solution. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution.
Acid Base TitrationWorking Principle, Process, Types And Indicators
Acid Base Titration Lab Procedure Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. See examples, definitions, and tips for using indicators and stoichiometry. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. For part a you will consider three types of reactions: The reagent (titrant) is the solution with a known molarity that will react with the analyte. Learn how to determine the concentration of unknown acids or bases by neutralizing them with a known solution. Follow the procedure for preparing solutions, standardizing naoh, and titrating khp with naoh. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or.
From
Acid Base Titration Lab Procedure Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Learn how to determine the concentration of unknown acids or bases by neutralizing them with a known solution. The concentration of a solution can be determined by knowing the acid and base dissociation constant. Follow the procedure for. Acid Base Titration Lab Procedure.
From
Acid Base Titration Lab Procedure See examples, definitions, and tips for using indicators and stoichiometry. For part a you will consider three types of reactions: Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. The concentration of a solution can be determined by knowing the acid and base dissociation constant. Learn how. Acid Base Titration Lab Procedure.
From
Acid Base Titration Lab Procedure For part a you will consider three types of reactions: See examples, definitions, and tips for using indicators and stoichiometry. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. Be able to determine the k a or k b from ph data associated with the titration of a. Acid Base Titration Lab Procedure.
From
Acid Base Titration Lab Procedure For part a you will consider three types of reactions: Follow the procedure for preparing solutions, standardizing naoh, and titrating khp with naoh. Learn how to determine the concentration of unknown acids or bases by neutralizing them with a known solution. The concentration of a solution can be determined by knowing the acid and base dissociation constant. A titration is. Acid Base Titration Lab Procedure.
From
Acid Base Titration Lab Procedure Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. See examples, definitions, and tips for using indicators and stoichiometry. For part a you will consider three types of reactions: A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with. Acid Base Titration Lab Procedure.
From
Acid Base Titration Lab Procedure Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. The analyte (titrand) is the solution with an unknown molarity. See examples, definitions, and tips for using. Acid Base Titration Lab Procedure.
From
Acid Base Titration Lab Procedure Learn how to determine the concentration of unknown acids or bases by neutralizing them with a known solution. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. Follow the procedure for preparing solutions, standardizing naoh, and titrating khp with naoh. See examples, definitions, and tips for using indicators. Acid Base Titration Lab Procedure.
From
Acid Base Titration Lab Procedure The reagent (titrant) is the solution with a known molarity that will react with the analyte. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. Learn how to determine the concentration of unknown acids or bases by neutralizing them with a known solution. Be able to determine the. Acid Base Titration Lab Procedure.
From stock.adobe.com
Acid base titration experiment and phases of color change during Acid Base Titration Lab Procedure For part a you will consider three types of reactions: The concentration of a solution can be determined by knowing the acid and base dissociation constant. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Follow the procedure for preparing solutions, standardizing naoh, and titrating khp with. Acid Base Titration Lab Procedure.
From
Acid Base Titration Lab Procedure Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. The concentration of a solution can be determined by knowing the acid and base dissociation constant. A. Acid Base Titration Lab Procedure.
From mungfali.com
Acid Base Titration Lab Acid Base Titration Lab Procedure For part a you will consider three types of reactions: Follow the procedure for preparing solutions, standardizing naoh, and titrating khp with naoh. The concentration of a solution can be determined by knowing the acid and base dissociation constant. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. Acid Base Titration Lab Procedure.
From letitsnowglobe.co.uk
Titration procedure pdf Acid Base Titration Lab Procedure Follow the procedure for preparing solutions, standardizing naoh, and titrating khp with naoh. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. See examples, definitions, and tips for using indicators and stoichiometry. Learn how to determine the concentration of unknown acids or bases by neutralizing them with. Acid Base Titration Lab Procedure.
From
Acid Base Titration Lab Procedure Follow the procedure for preparing solutions, standardizing naoh, and titrating khp with naoh. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation. Acid Base Titration Lab Procedure.
From
Acid Base Titration Lab Procedure Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. For part a you will consider three types of reactions: The analyte (titrand) is the solution with an unknown molarity. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein. Acid Base Titration Lab Procedure.
From
Acid Base Titration Lab Procedure The reagent (titrant) is the solution with a known molarity that will react with the analyte. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid. Acid Base Titration Lab Procedure.
From mungfali.com
Acid Base Titration Lab Acid Base Titration Lab Procedure A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. The concentration of a solution can be determined by knowing the acid and base dissociation constant. See examples, definitions, and tips for using indicators and stoichiometry. Learn how to perform a titration to determine the concentration of an. Acid Base Titration Lab Procedure.
From www.slideserve.com
PPT AcidBase Titration and pH PowerPoint Presentation, free download Acid Base Titration Lab Procedure For part a you will consider three types of reactions: A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Learn how to determine the concentration of unknown acids or bases by neutralizing them with a known solution. See examples, definitions, and tips for using indicators and stoichiometry.. Acid Base Titration Lab Procedure.
From
Acid Base Titration Lab Procedure The analyte (titrand) is the solution with an unknown molarity. For part a you will consider three types of reactions: Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. See examples, definitions, and tips for using indicators and stoichiometry. A titration is an analytical procedure used to determine. Acid Base Titration Lab Procedure.
From
Acid Base Titration Lab Procedure See examples, definitions, and tips for using indicators and stoichiometry. For part a you will consider three types of reactions: Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. Follow the procedure for preparing solutions, standardizing naoh, and titrating khp with naoh. Be able to determine the k. Acid Base Titration Lab Procedure.
From
Acid Base Titration Lab Procedure See examples, definitions, and tips for using indicators and stoichiometry. The reagent (titrant) is the solution with a known molarity that will react with the analyte. Learn how to determine the concentration of unknown acids or bases by neutralizing them with a known solution. Be able to determine the k a or k b from ph data associated with the. Acid Base Titration Lab Procedure.
From
Acid Base Titration Lab Procedure For part a you will consider three types of reactions: Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. The concentration of a solution can be determined by knowing the acid and base dissociation constant. A titration is an analytical procedure used to determine the accurate concentration of. Acid Base Titration Lab Procedure.
From
Acid Base Titration Lab Procedure Learn how to determine the concentration of unknown acids or bases by neutralizing them with a known solution. The reagent (titrant) is the solution with a known molarity that will react with the analyte. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. A titration is an. Acid Base Titration Lab Procedure.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Acid Base Titration Lab Procedure See examples, definitions, and tips for using indicators and stoichiometry. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. The concentration of a solution can be determined by knowing the acid and base dissociation constant. Follow the procedure for preparing solutions, standardizing naoh, and titrating khp with naoh.. Acid Base Titration Lab Procedure.
From
Acid Base Titration Lab Procedure A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Learn how to determine the concentration of unknown acids or bases by neutralizing them with a known solution. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an. Acid Base Titration Lab Procedure.
From mungfali.com
Acid Base Titration Procedure Acid Base Titration Lab Procedure A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Learn how to determine the concentration of unknown acids or bases by neutralizing them with a known solution. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an. Acid Base Titration Lab Procedure.
From mungfali.com
Acid Base Titration Procedure Acid Base Titration Lab Procedure Learn how to determine the concentration of unknown acids or bases by neutralizing them with a known solution. The concentration of a solution can be determined by knowing the acid and base dissociation constant. See examples, definitions, and tips for using indicators and stoichiometry. For part a you will consider three types of reactions: Follow the procedure for preparing solutions,. Acid Base Titration Lab Procedure.
From mungfali.com
Acid Base Titration Lab Procedure Acid Base Titration Lab Procedure Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. For part a you will consider three types of reactions: Follow the. Acid Base Titration Lab Procedure.
From
Acid Base Titration Lab Procedure The reagent (titrant) is the solution with a known molarity that will react with the analyte. Learn how to determine the concentration of unknown acids or bases by neutralizing them with a known solution. The concentration of a solution can be determined by knowing the acid and base dissociation constant. A titration is an analytical procedure used to determine the. Acid Base Titration Lab Procedure.
From
Acid Base Titration Lab Procedure The reagent (titrant) is the solution with a known molarity that will react with the analyte. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. Learn how to determine the concentration of unknown acids or bases by neutralizing them with a known solution. Follow the procedure for preparing. Acid Base Titration Lab Procedure.
From
Acid Base Titration Lab Procedure For part a you will consider three types of reactions: Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The analyte (titrand) is the solution with an unknown molarity. Follow the. Acid Base Titration Lab Procedure.
From
Acid Base Titration Lab Procedure Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Learn how to determine the concentration of unknown acids or bases by neutralizing them with a known solution. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an. Acid Base Titration Lab Procedure.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Acid Base Titration Lab Procedure The reagent (titrant) is the solution with a known molarity that will react with the analyte. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. See examples, definitions, and tips for using indicators and stoichiometry. The analyte (titrand) is the solution with an unknown molarity. Learn how. Acid Base Titration Lab Procedure.
From mavink.com
Acid Base Titration Lab Procedure Acid Base Titration Lab Procedure See examples, definitions, and tips for using indicators and stoichiometry. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Follow the procedure for preparing solutions, standardizing naoh, and titrating khp with naoh. For part a you will consider three types of reactions: The analyte (titrand) is the. Acid Base Titration Lab Procedure.
From
Acid Base Titration Lab Procedure Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Learn how to determine the concentration of unknown acids or bases by neutralizing them with a known solution. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a. Acid Base Titration Lab Procedure.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Acid Base Titration Lab Procedure The concentration of a solution can be determined by knowing the acid and base dissociation constant. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. Learn how to determine the concentration of unknown acids or bases by neutralizing them with a known solution. Be able to determine the. Acid Base Titration Lab Procedure.