Properties Of Water Model 1 . The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel one another and the molecule as a whole is polar. Even if water might seem boring to you—no color, taste, or. The presence and number of hydrogen bonds between water molecules;. Without it every bodies (82% of our blood is water). The water molecule, visualized three different ways: The physical and chemical properties of water are what make it essential to life and useful to civilization. Water has many essential roles in living organisms due to its properties: The polarity of water molecules; Water is fundamental for all life; One of the most important chemical properties of water is its ability to behave as both an acid (a proton donor) and a base (a proton.
from www.worksheetsplanet.com
The presence and number of hydrogen bonds between water molecules;. One of the most important chemical properties of water is its ability to behave as both an acid (a proton donor) and a base (a proton. The water molecule, visualized three different ways: Water has many essential roles in living organisms due to its properties: Even if water might seem boring to you—no color, taste, or. The physical and chemical properties of water are what make it essential to life and useful to civilization. The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel one another and the molecule as a whole is polar. Without it every bodies (82% of our blood is water). The polarity of water molecules; Water is fundamental for all life;
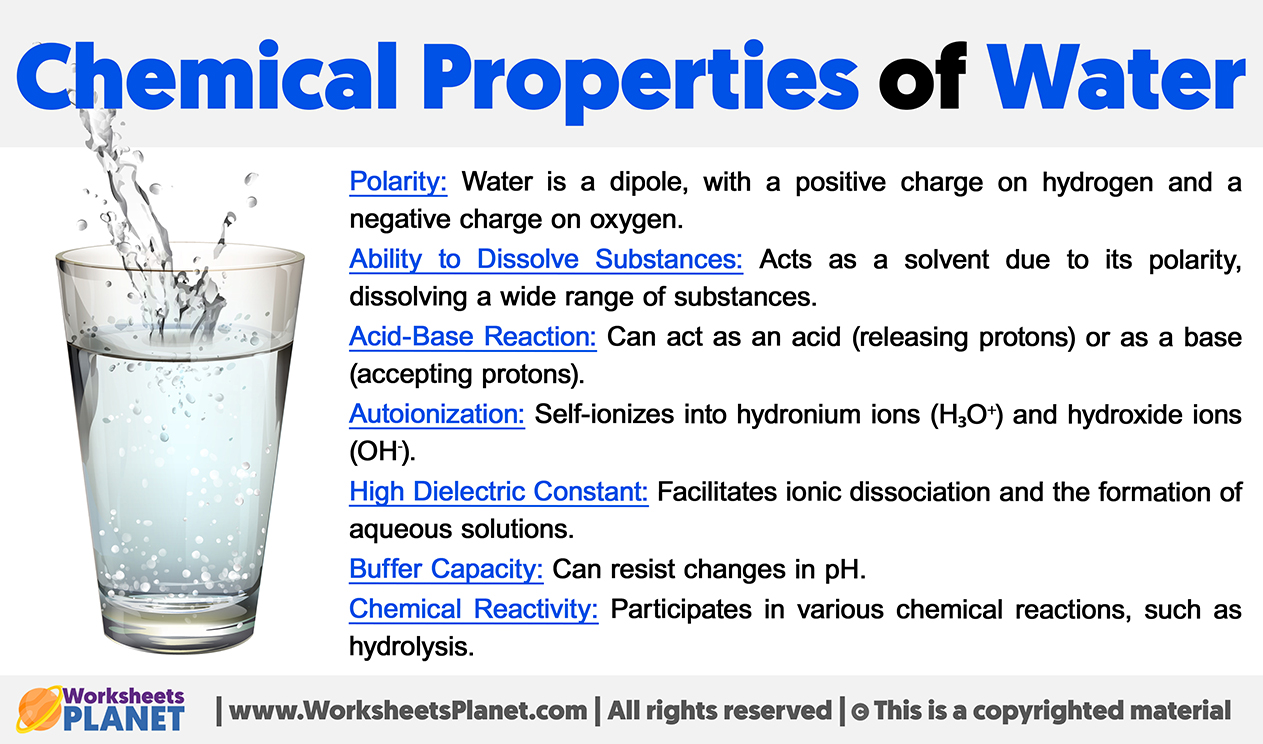
Chemical Properties of Water
Properties Of Water Model 1 The polarity of water molecules; The water molecule, visualized three different ways: Without it every bodies (82% of our blood is water). The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel one another and the molecule as a whole is polar. Even if water might seem boring to you—no color, taste, or. One of the most important chemical properties of water is its ability to behave as both an acid (a proton donor) and a base (a proton. The presence and number of hydrogen bonds between water molecules;. Water has many essential roles in living organisms due to its properties: The polarity of water molecules; The physical and chemical properties of water are what make it essential to life and useful to civilization. Water is fundamental for all life;
From www.youtube.com
Sources Of Water Model For School Project Water Resources Model Properties Of Water Model 1 The water molecule, visualized three different ways: Water has many essential roles in living organisms due to its properties: The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel one another and the molecule as a whole is polar. Even if water might seem boring to you—no color, taste, or.. Properties Of Water Model 1.
From studylib.net
Introduction to the Properties of Water What Makes Water So Special Properties Of Water Model 1 The presence and number of hydrogen bonds between water molecules;. Water is fundamental for all life; The water molecule, visualized three different ways: Even if water might seem boring to you—no color, taste, or. Without it every bodies (82% of our blood is water). One of the most important chemical properties of water is its ability to behave as both. Properties Of Water Model 1.
From slideplayer.com
Properties of Water Essential Questions ppt download Properties Of Water Model 1 Even if water might seem boring to you—no color, taste, or. The presence and number of hydrogen bonds between water molecules;. The polarity of water molecules; The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel one another and the molecule as a whole is polar. The physical and chemical. Properties Of Water Model 1.
From www.youtube.com
Properties of Water AP Bio Topic 1.1 YouTube Properties Of Water Model 1 The polarity of water molecules; Even if water might seem boring to you—no color, taste, or. Water has many essential roles in living organisms due to its properties: The presence and number of hydrogen bonds between water molecules;. Without it every bodies (82% of our blood is water). One of the most important chemical properties of water is its ability. Properties Of Water Model 1.
From www.eurekalert.org
Water models for the accurate [IMAGE] EurekAlert! Science News Releases Properties Of Water Model 1 Without it every bodies (82% of our blood is water). Water is fundamental for all life; The polarity of water molecules; The presence and number of hydrogen bonds between water molecules;. Even if water might seem boring to you—no color, taste, or. The water molecule, visualized three different ways: Water has many essential roles in living organisms due to its. Properties Of Water Model 1.
From www.youtube.com
Properties of Water For Kids Science Tutway YouTube Properties Of Water Model 1 The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel one another and the molecule as a whole is polar. The presence and number of hydrogen bonds between water molecules;. Water has many essential roles in living organisms due to its properties: Without it every bodies (82% of our blood. Properties Of Water Model 1.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Properties Of Water Model 1 One of the most important chemical properties of water is its ability to behave as both an acid (a proton donor) and a base (a proton. Water has many essential roles in living organisms due to its properties: The water molecule, visualized three different ways: Water is fundamental for all life; The physical and chemical properties of water are what. Properties Of Water Model 1.
From www.youtube.com
Sources Of Water Model For School Project Water Resources Model Properties Of Water Model 1 Even if water might seem boring to you—no color, taste, or. One of the most important chemical properties of water is its ability to behave as both an acid (a proton donor) and a base (a proton. Water is fundamental for all life; Water has many essential roles in living organisms due to its properties: Without it every bodies (82%. Properties Of Water Model 1.
From www.researchgate.net
Anatomy of surfacewater model Download Scientific Diagram Properties Of Water Model 1 Water has many essential roles in living organisms due to its properties: Even if water might seem boring to you—no color, taste, or. One of the most important chemical properties of water is its ability to behave as both an acid (a proton donor) and a base (a proton. Water is fundamental for all life; Without it every bodies (82%. Properties Of Water Model 1.
From www.compchem.nl
Development of Classical WaterModels for the Computer Simulation of Properties Of Water Model 1 The physical and chemical properties of water are what make it essential to life and useful to civilization. Water has many essential roles in living organisms due to its properties: The presence and number of hydrogen bonds between water molecules;. The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel. Properties Of Water Model 1.
From studylib.net
Guided Notes Properties of Water Properties Of Water Model 1 The physical and chemical properties of water are what make it essential to life and useful to civilization. Water has many essential roles in living organisms due to its properties: Even if water might seem boring to you—no color, taste, or. One of the most important chemical properties of water is its ability to behave as both an acid (a. Properties Of Water Model 1.
From www.studocu.com
5 Properties of WaterS Properties of 1 Oxygen Covalent Hydrogen Properties Of Water Model 1 The water molecule, visualized three different ways: The presence and number of hydrogen bonds between water molecules;. One of the most important chemical properties of water is its ability to behave as both an acid (a proton donor) and a base (a proton. The physical and chemical properties of water are what make it essential to life and useful to. Properties Of Water Model 1.
From www.worksheetsplanet.com
Chemical Properties of Water Properties Of Water Model 1 The polarity of water molecules; The presence and number of hydrogen bonds between water molecules;. Without it every bodies (82% of our blood is water). Water has many essential roles in living organisms due to its properties: One of the most important chemical properties of water is its ability to behave as both an acid (a proton donor) and a. Properties Of Water Model 1.
From animalia-life.club
Properties Of Water Polarity Properties Of Water Model 1 Without it every bodies (82% of our blood is water). Water has many essential roles in living organisms due to its properties: The water molecule, visualized three different ways: The physical and chemical properties of water are what make it essential to life and useful to civilization. Even if water might seem boring to you—no color, taste, or. One of. Properties Of Water Model 1.
From www.slideserve.com
PPT Biophysical Chemistry G4170 Introduction to Molecular Dynamics Properties Of Water Model 1 Water has many essential roles in living organisms due to its properties: Without it every bodies (82% of our blood is water). Water is fundamental for all life; The physical and chemical properties of water are what make it essential to life and useful to civilization. One of the most important chemical properties of water is its ability to behave. Properties Of Water Model 1.
From mungfali.com
Lewis Structure Of Water Properties Of Water Model 1 One of the most important chemical properties of water is its ability to behave as both an acid (a proton donor) and a base (a proton. The physical and chemical properties of water are what make it essential to life and useful to civilization. The bent shape of the water molecule is critical because the polar o−h o − h. Properties Of Water Model 1.
From iteachly.com
Properties of Water Biology Activities ⋆ Properties Of Water Model 1 Without it every bodies (82% of our blood is water). The polarity of water molecules; The water molecule, visualized three different ways: The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel one another and the molecule as a whole is polar. The presence and number of hydrogen bonds between. Properties Of Water Model 1.
From www.youtube.com
Sources of Water Model Science Project School Project DIY 3D Properties Of Water Model 1 Without it every bodies (82% of our blood is water). The polarity of water molecules; Even if water might seem boring to you—no color, taste, or. The water molecule, visualized three different ways: Water is fundamental for all life; The presence and number of hydrogen bonds between water molecules;. The bent shape of the water molecule is critical because the. Properties Of Water Model 1.
From studylib.net
Properties of Water Parts I & II Properties Of Water Model 1 Water has many essential roles in living organisms due to its properties: The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel one another and the molecule as a whole is polar. Without it every bodies (82% of our blood is water). Even if water might seem boring to you—no. Properties Of Water Model 1.
From studylib.net
Properties of Water Properties Of Water Model 1 One of the most important chemical properties of water is its ability to behave as both an acid (a proton donor) and a base (a proton. Water has many essential roles in living organisms due to its properties: Water is fundamental for all life; Without it every bodies (82% of our blood is water). The bent shape of the water. Properties Of Water Model 1.
From owlcation.com
5 Properties of Water Owlcation Properties Of Water Model 1 The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel one another and the molecule as a whole is polar. The polarity of water molecules; Without it every bodies (82% of our blood is water). The water molecule, visualized three different ways: Water has many essential roles in living organisms. Properties Of Water Model 1.
From www.slideserve.com
PPT The Chemistry of Life PowerPoint Presentation, free download ID Properties Of Water Model 1 Water has many essential roles in living organisms due to its properties: Water is fundamental for all life; Without it every bodies (82% of our blood is water). The presence and number of hydrogen bonds between water molecules;. The polarity of water molecules; The water molecule, visualized three different ways: The bent shape of the water molecule is critical because. Properties Of Water Model 1.
From studylib.net
Properties of Water Properties Of Water Model 1 Water is fundamental for all life; The presence and number of hydrogen bonds between water molecules;. Without it every bodies (82% of our blood is water). The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel one another and the molecule as a whole is polar. One of the most. Properties Of Water Model 1.
From thehappyhousewife.com
Properties of Water Experiments The Happy Housewife™ Home Schooling Properties Of Water Model 1 The water molecule, visualized three different ways: The physical and chemical properties of water are what make it essential to life and useful to civilization. Water is fundamental for all life; Without it every bodies (82% of our blood is water). Water has many essential roles in living organisms due to its properties: One of the most important chemical properties. Properties Of Water Model 1.
From teachingideas.ca
Properties of Water for Kids HandsOn Teaching Ideas HandsOn Fun Properties Of Water Model 1 The presence and number of hydrogen bonds between water molecules;. The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel one another and the molecule as a whole is polar. Water is fundamental for all life; Water has many essential roles in living organisms due to its properties: The polarity. Properties Of Water Model 1.
From www.youtube.com
AP Biology Properties of Water YouTube Properties Of Water Model 1 The polarity of water molecules; The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel one another and the molecule as a whole is polar. Without it every bodies (82% of our blood is water). Water has many essential roles in living organisms due to its properties: Water is fundamental. Properties Of Water Model 1.
From emmatheteachie.com
Properties of Water Bundle Emmatheteachie Properties Of Water Model 1 One of the most important chemical properties of water is its ability to behave as both an acid (a proton donor) and a base (a proton. Even if water might seem boring to you—no color, taste, or. Water is fundamental for all life; Without it every bodies (82% of our blood is water). Water has many essential roles in living. Properties Of Water Model 1.
From enjoy-teaching.com
Properties of Water Lesson Plans Fun Science Activities Properties Of Water Model 1 The polarity of water molecules; The physical and chemical properties of water are what make it essential to life and useful to civilization. The water molecule, visualized three different ways: The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel one another and the molecule as a whole is polar.. Properties Of Water Model 1.
From animalia-life.club
Properties Of Water Polarity Properties Of Water Model 1 The polarity of water molecules; The water molecule, visualized three different ways: The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel one another and the molecule as a whole is polar. Water is fundamental for all life; The presence and number of hydrogen bonds between water molecules;. Even if. Properties Of Water Model 1.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Properties Of Water Model 1 The polarity of water molecules; The presence and number of hydrogen bonds between water molecules;. Without it every bodies (82% of our blood is water). One of the most important chemical properties of water is its ability to behave as both an acid (a proton donor) and a base (a proton. Water is fundamental for all life; Even if water. Properties Of Water Model 1.
From www.nagwa.com
Lesson Video Properties of Water Nagwa Properties Of Water Model 1 The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel one another and the molecule as a whole is polar. The water molecule, visualized three different ways: Even if water might seem boring to you—no color, taste, or. The polarity of water molecules; The presence and number of hydrogen bonds. Properties Of Water Model 1.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Properties Of Water Model 1 Water has many essential roles in living organisms due to its properties: Water is fundamental for all life; Without it every bodies (82% of our blood is water). The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel one another and the molecule as a whole is polar. One of. Properties Of Water Model 1.
From www.worksheetsplanet.com
Physical Properties of Water Properties Of Water Model 1 Water is fundamental for all life; The polarity of water molecules; Without it every bodies (82% of our blood is water). The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel one another and the molecule as a whole is polar. Even if water might seem boring to you—no color,. Properties Of Water Model 1.
From chemistrytalk.org
Physical & Chemical Properties of Water ChemTalk Properties Of Water Model 1 Without it every bodies (82% of our blood is water). One of the most important chemical properties of water is its ability to behave as both an acid (a proton donor) and a base (a proton. Water is fundamental for all life; The polarity of water molecules; The physical and chemical properties of water are what make it essential to. Properties Of Water Model 1.
From courses.lumenlearning.com
The Structure and Properties of Water Introduction to Chemistry Properties Of Water Model 1 Water has many essential roles in living organisms due to its properties: The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel one another and the molecule as a whole is polar. The physical and chemical properties of water are what make it essential to life and useful to civilization.. Properties Of Water Model 1.