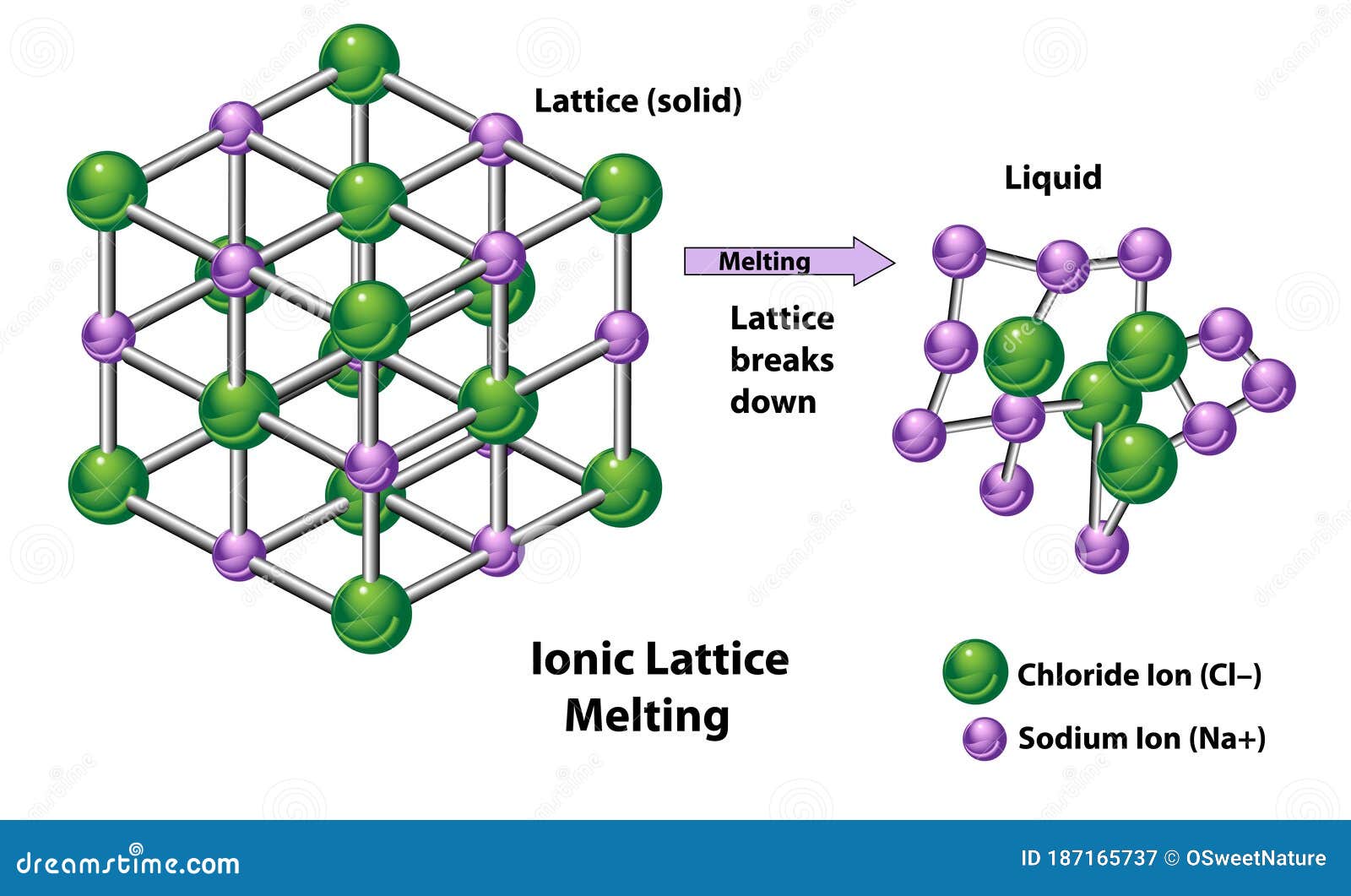
Ionic Crystal Lattice . A ball and stick model makes it easier to see how individual ions are oriented with respect to one. Explain what features of a crystal are reflected in its madelung. The regular arrangement of ions in an ionic substance. \(u\) can be calculated from the charges on the ions, the arrangement of the ions in the solid, and the internuclear distance. The lattice is formed because the ions attract each other and form a regular. Ionic crystals are arranged in a lattice structure of positive and negative ions. The lattice structure is the responsible of the crystalline nature of ionic. Many ionic compounds crystallize with cubic unit cells, and we will use these compounds to describe the general features of ionic. The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; The figure below shows two different ways of representing the ionic crystal lattice. List the properties of ionic crystals, and relate them to the lattice energy.
from www.vrogue.co
The lattice structure is the responsible of the crystalline nature of ionic. List the properties of ionic crystals, and relate them to the lattice energy. The regular arrangement of ions in an ionic substance. The lattice is formed because the ions attract each other and form a regular. \(u\) can be calculated from the charges on the ions, the arrangement of the ions in the solid, and the internuclear distance. A ball and stick model makes it easier to see how individual ions are oriented with respect to one. The figure below shows two different ways of representing the ionic crystal lattice. Ionic crystals are arranged in a lattice structure of positive and negative ions. Many ionic compounds crystallize with cubic unit cells, and we will use these compounds to describe the general features of ionic. Explain what features of a crystal are reflected in its madelung.
The Crystal Lattice Structure Of Ionic Compounds Info vrogue.co
Ionic Crystal Lattice \(u\) can be calculated from the charges on the ions, the arrangement of the ions in the solid, and the internuclear distance. The lattice is formed because the ions attract each other and form a regular. A ball and stick model makes it easier to see how individual ions are oriented with respect to one. The figure below shows two different ways of representing the ionic crystal lattice. List the properties of ionic crystals, and relate them to the lattice energy. The regular arrangement of ions in an ionic substance. The lattice structure is the responsible of the crystalline nature of ionic. Ionic crystals are arranged in a lattice structure of positive and negative ions. The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; Many ionic compounds crystallize with cubic unit cells, and we will use these compounds to describe the general features of ionic. \(u\) can be calculated from the charges on the ions, the arrangement of the ions in the solid, and the internuclear distance. Explain what features of a crystal are reflected in its madelung.
From www.slideserve.com
PPT Ionic Bonding and Ionic Compounds PowerPoint Presentation, free Ionic Crystal Lattice The lattice is formed because the ions attract each other and form a regular. The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; List the properties of ionic crystals, and relate them to the lattice energy. The lattice structure is the responsible of the crystalline nature of ionic.. Ionic Crystal Lattice.
From www.shutterstock.com
Vektor Stok Crystal Lattice Structure Ionic Compounds Ionic (Tanpa Ionic Crystal Lattice The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; List the properties of ionic crystals, and relate them to the lattice energy. \(u\) can be calculated from the charges on the ions, the arrangement of the ions in the solid, and the internuclear distance. A ball and stick. Ionic Crystal Lattice.
From courses.lumenlearning.com
Ionic Crystals Introduction to Chemistry Ionic Crystal Lattice Ionic crystals are arranged in a lattice structure of positive and negative ions. \(u\) can be calculated from the charges on the ions, the arrangement of the ions in the solid, and the internuclear distance. A ball and stick model makes it easier to see how individual ions are oriented with respect to one. The lattice energy (\(u\)) of an. Ionic Crystal Lattice.
From en.wikipedia.org
Ionic compound Wikipedia Ionic Crystal Lattice List the properties of ionic crystals, and relate them to the lattice energy. The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; A ball and stick model makes it easier to see how individual ions are oriented with respect to one. The lattice structure is the responsible of. Ionic Crystal Lattice.
From chem.libretexts.org
Chapter 4.2 Lattice Energies in Ionic Solids Chemistry LibreTexts Ionic Crystal Lattice Explain what features of a crystal are reflected in its madelung. List the properties of ionic crystals, and relate them to the lattice energy. Many ionic compounds crystallize with cubic unit cells, and we will use these compounds to describe the general features of ionic. A ball and stick model makes it easier to see how individual ions are oriented. Ionic Crystal Lattice.
From www.slideserve.com
PPT Ionic bondscrystalline structure PowerPoint Presentation, free Ionic Crystal Lattice The lattice is formed because the ions attract each other and form a regular. The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; The regular arrangement of ions in an ionic substance. Many ionic compounds crystallize with cubic unit cells, and we will use these compounds to describe. Ionic Crystal Lattice.
From www.slideserve.com
PPT Ionic Bonds PowerPoint Presentation, free download ID1990542 Ionic Crystal Lattice The regular arrangement of ions in an ionic substance. Explain what features of a crystal are reflected in its madelung. Many ionic compounds crystallize with cubic unit cells, and we will use these compounds to describe the general features of ionic. \(u\) can be calculated from the charges on the ions, the arrangement of the ions in the solid, and. Ionic Crystal Lattice.
From slidetodoc.com
GIANT IONIC LATTICES Ionic Bonding the net electrostatic Ionic Crystal Lattice The figure below shows two different ways of representing the ionic crystal lattice. \(u\) can be calculated from the charges on the ions, the arrangement of the ions in the solid, and the internuclear distance. A ball and stick model makes it easier to see how individual ions are oriented with respect to one. Many ionic compounds crystallize with cubic. Ionic Crystal Lattice.
From www.vrogue.co
The Crystal Lattice Structure Of Ionic Compounds Info vrogue.co Ionic Crystal Lattice Many ionic compounds crystallize with cubic unit cells, and we will use these compounds to describe the general features of ionic. The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; Explain what features of a crystal are reflected in its madelung. Ionic crystals are arranged in a lattice. Ionic Crystal Lattice.
From www.slideserve.com
PPT Properties of ionic compounds PowerPoint Presentation, free Ionic Crystal Lattice Ionic crystals are arranged in a lattice structure of positive and negative ions. \(u\) can be calculated from the charges on the ions, the arrangement of the ions in the solid, and the internuclear distance. The regular arrangement of ions in an ionic substance. A ball and stick model makes it easier to see how individual ions are oriented with. Ionic Crystal Lattice.
From www.thesciencehive.co.uk
Ionic Bonding — the science hive Ionic Crystal Lattice The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; The regular arrangement of ions in an ionic substance. List the properties of ionic crystals, and relate them to the lattice energy. The figure below shows two different ways of representing the ionic crystal lattice. \(u\) can be calculated. Ionic Crystal Lattice.
From www.vrogue.co
The Crystal Lattice Structure Of Ionic Compounds Info vrogue.co Ionic Crystal Lattice A ball and stick model makes it easier to see how individual ions are oriented with respect to one. Explain what features of a crystal are reflected in its madelung. Ionic crystals are arranged in a lattice structure of positive and negative ions. The figure below shows two different ways of representing the ionic crystal lattice. \(u\) can be calculated. Ionic Crystal Lattice.
From www.vrogue.co
The Crystal Lattice Structure Of Ionic Compounds Info vrogue.co Ionic Crystal Lattice A ball and stick model makes it easier to see how individual ions are oriented with respect to one. The lattice structure is the responsible of the crystalline nature of ionic. The regular arrangement of ions in an ionic substance. \(u\) can be calculated from the charges on the ions, the arrangement of the ions in the solid, and the. Ionic Crystal Lattice.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Ionic Crystal Lattice The lattice is formed because the ions attract each other and form a regular. Explain what features of a crystal are reflected in its madelung. The lattice structure is the responsible of the crystalline nature of ionic. The regular arrangement of ions in an ionic substance. \(u\) can be calculated from the charges on the ions, the arrangement of the. Ionic Crystal Lattice.
From psiberg.com
Types of Crystalline Solids The Seven Crystal System Ionic Crystal Lattice \(u\) can be calculated from the charges on the ions, the arrangement of the ions in the solid, and the internuclear distance. Ionic crystals are arranged in a lattice structure of positive and negative ions. Many ionic compounds crystallize with cubic unit cells, and we will use these compounds to describe the general features of ionic. The lattice energy (\(u\)). Ionic Crystal Lattice.
From www.alamy.com
Caesium chloride crystal lattice ionic structure, illustration Stock Ionic Crystal Lattice \(u\) can be calculated from the charges on the ions, the arrangement of the ions in the solid, and the internuclear distance. The lattice structure is the responsible of the crystalline nature of ionic. The lattice is formed because the ions attract each other and form a regular. List the properties of ionic crystals, and relate them to the lattice. Ionic Crystal Lattice.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica Ionic Crystal Lattice List the properties of ionic crystals, and relate them to the lattice energy. Ionic crystals are arranged in a lattice structure of positive and negative ions. \(u\) can be calculated from the charges on the ions, the arrangement of the ions in the solid, and the internuclear distance. The lattice structure is the responsible of the crystalline nature of ionic.. Ionic Crystal Lattice.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Ionic Crystal Lattice \(u\) can be calculated from the charges on the ions, the arrangement of the ions in the solid, and the internuclear distance. The figure below shows two different ways of representing the ionic crystal lattice. The lattice structure is the responsible of the crystalline nature of ionic. List the properties of ionic crystals, and relate them to the lattice energy.. Ionic Crystal Lattice.
From courses.lumenlearning.com
Ionic Crystals Introduction to Chemistry Ionic Crystal Lattice \(u\) can be calculated from the charges on the ions, the arrangement of the ions in the solid, and the internuclear distance. The lattice is formed because the ions attract each other and form a regular. Ionic crystals are arranged in a lattice structure of positive and negative ions. List the properties of ionic crystals, and relate them to the. Ionic Crystal Lattice.
From mungfali.com
Ionic Compound Structure Ionic Crystal Lattice The figure below shows two different ways of representing the ionic crystal lattice. The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; The regular arrangement of ions in an ionic substance. The lattice is formed because the ions attract each other and form a regular. The lattice structure. Ionic Crystal Lattice.
From www.vrogue.co
The Crystal Lattice Structure Of Ionic Compounds Info vrogue.co Ionic Crystal Lattice List the properties of ionic crystals, and relate them to the lattice energy. The lattice structure is the responsible of the crystalline nature of ionic. The lattice is formed because the ions attract each other and form a regular. Many ionic compounds crystallize with cubic unit cells, and we will use these compounds to describe the general features of ionic.. Ionic Crystal Lattice.
From www.vrogue.co
The Crystal Lattice Structure Of Ionic Compounds Info vrogue.co Ionic Crystal Lattice Many ionic compounds crystallize with cubic unit cells, and we will use these compounds to describe the general features of ionic. The lattice structure is the responsible of the crystalline nature of ionic. A ball and stick model makes it easier to see how individual ions are oriented with respect to one. Explain what features of a crystal are reflected. Ionic Crystal Lattice.
From www.vrogue.co
The Crystal Lattice Structure Of Ionic Compounds Info vrogue.co Ionic Crystal Lattice The lattice is formed because the ions attract each other and form a regular. Explain what features of a crystal are reflected in its madelung. The figure below shows two different ways of representing the ionic crystal lattice. The lattice structure is the responsible of the crystalline nature of ionic. The regular arrangement of ions in an ionic substance. Ionic. Ionic Crystal Lattice.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Ionic Crystal Lattice Ionic crystals are arranged in a lattice structure of positive and negative ions. The figure below shows two different ways of representing the ionic crystal lattice. List the properties of ionic crystals, and relate them to the lattice energy. The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions;. Ionic Crystal Lattice.
From keystagewiki.com
Giant Ionic Structure Key Stage Wiki Ionic Crystal Lattice The figure below shows two different ways of representing the ionic crystal lattice. Ionic crystals are arranged in a lattice structure of positive and negative ions. The lattice is formed because the ions attract each other and form a regular. The lattice structure is the responsible of the crystalline nature of ionic. A ball and stick model makes it easier. Ionic Crystal Lattice.
From www.dreamstime.com
Ionic Lattice Model of Sodium and Chloride Molecules Stock Vector Ionic Crystal Lattice The regular arrangement of ions in an ionic substance. A ball and stick model makes it easier to see how individual ions are oriented with respect to one. Many ionic compounds crystallize with cubic unit cells, and we will use these compounds to describe the general features of ionic. The figure below shows two different ways of representing the ionic. Ionic Crystal Lattice.
From www.slideserve.com
PPT Ionic Bonding, NaCl PowerPoint Presentation, free download ID Ionic Crystal Lattice \(u\) can be calculated from the charges on the ions, the arrangement of the ions in the solid, and the internuclear distance. Explain what features of a crystal are reflected in its madelung. Ionic crystals are arranged in a lattice structure of positive and negative ions. The figure below shows two different ways of representing the ionic crystal lattice. A. Ionic Crystal Lattice.
From www.savemyexams.com
Ionic Lattices SL IB Chemistry Revision Notes 2025 Ionic Crystal Lattice List the properties of ionic crystals, and relate them to the lattice energy. Many ionic compounds crystallize with cubic unit cells, and we will use these compounds to describe the general features of ionic. The regular arrangement of ions in an ionic substance. Explain what features of a crystal are reflected in its madelung. The figure below shows two different. Ionic Crystal Lattice.
From studylib.net
The Crystal Lattice Ionic Crystal Lattice List the properties of ionic crystals, and relate them to the lattice energy. Ionic crystals are arranged in a lattice structure of positive and negative ions. Explain what features of a crystal are reflected in its madelung. The lattice structure is the responsible of the crystalline nature of ionic. The figure below shows two different ways of representing the ionic. Ionic Crystal Lattice.
From www.graylark.com
Ionic Solids Ionic Crystal Lattice A ball and stick model makes it easier to see how individual ions are oriented with respect to one. Ionic crystals are arranged in a lattice structure of positive and negative ions. Many ionic compounds crystallize with cubic unit cells, and we will use these compounds to describe the general features of ionic. The lattice structure is the responsible of. Ionic Crystal Lattice.
From philschatz.com
Lattice Structures in Crystalline Solids · Chemistry Ionic Crystal Lattice A ball and stick model makes it easier to see how individual ions are oriented with respect to one. The lattice is formed because the ions attract each other and form a regular. List the properties of ionic crystals, and relate them to the lattice energy. Ionic crystals are arranged in a lattice structure of positive and negative ions. Explain. Ionic Crystal Lattice.
From chemistnotes.com
Ionic Crystal, Examples, and Properties Chemistry Notes Ionic Crystal Lattice List the properties of ionic crystals, and relate them to the lattice energy. \(u\) can be calculated from the charges on the ions, the arrangement of the ions in the solid, and the internuclear distance. The regular arrangement of ions in an ionic substance. Ionic crystals are arranged in a lattice structure of positive and negative ions. Explain what features. Ionic Crystal Lattice.
From www.britannica.com
Crystal Structure, Lattice, Symmetry Britannica Ionic Crystal Lattice \(u\) can be calculated from the charges on the ions, the arrangement of the ions in the solid, and the internuclear distance. Ionic crystals are arranged in a lattice structure of positive and negative ions. Explain what features of a crystal are reflected in its madelung. A ball and stick model makes it easier to see how individual ions are. Ionic Crystal Lattice.
From slidetodoc.com
GIANT IONIC LATTICES Ionic Bonding the net electrostatic Ionic Crystal Lattice Explain what features of a crystal are reflected in its madelung. Many ionic compounds crystallize with cubic unit cells, and we will use these compounds to describe the general features of ionic. The regular arrangement of ions in an ionic substance. List the properties of ionic crystals, and relate them to the lattice energy. The lattice energy (\(u\)) of an. Ionic Crystal Lattice.
From www.alamy.com
Ionic crystal lattice model Stock Vector Images Alamy Ionic Crystal Lattice A ball and stick model makes it easier to see how individual ions are oriented with respect to one. The figure below shows two different ways of representing the ionic crystal lattice. The lattice is formed because the ions attract each other and form a regular. \(u\) can be calculated from the charges on the ions, the arrangement of the. Ionic Crystal Lattice.