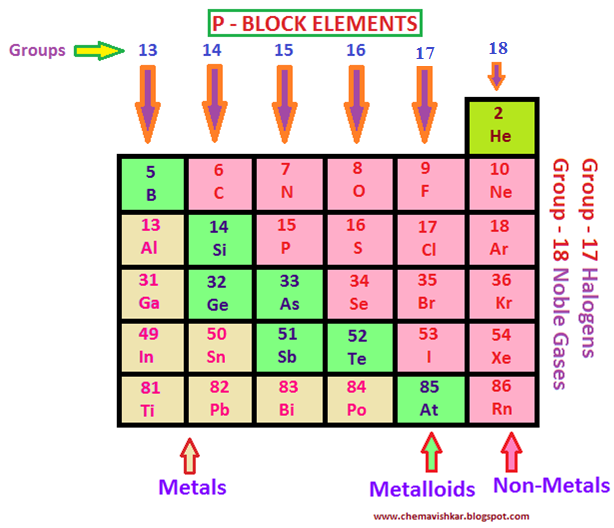
P Block Periodic Table Of Elements . The “p” refers to the principal and azimuthal quantum number 1. They include the last six element groups of the table (except for helium). Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. The maximum number of electrons that can occur in a set of p orbitals is six since there are three p orbitals. This block is situated at the extreme right of the periodic table. Interact with other chemicals by losing, gaining, or sharing the valence electrons. They get their name from the fact that their valence electrons, or the electrons in the outermost shell, reside in the p orbital.
from chemavishkar.blogspot.com
Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. The “p” refers to the principal and azimuthal quantum number 1. This block is situated at the extreme right of the periodic table. The maximum number of electrons that can occur in a set of p orbitals is six since there are three p orbitals. Interact with other chemicals by losing, gaining, or sharing the valence electrons. They get their name from the fact that their valence electrons, or the electrons in the outermost shell, reside in the p orbital. They include the last six element groups of the table (except for helium).
PBLOCK ELEMENTS OF THE MODERN PERIODIC TABLE. What are P BLOCK
P Block Periodic Table Of Elements They get their name from the fact that their valence electrons, or the electrons in the outermost shell, reside in the p orbital. This block is situated at the extreme right of the periodic table. Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. They include the last six element groups of the table (except for helium). The “p” refers to the principal and azimuthal quantum number 1. Interact with other chemicals by losing, gaining, or sharing the valence electrons. They get their name from the fact that their valence electrons, or the electrons in the outermost shell, reside in the p orbital. The maximum number of electrons that can occur in a set of p orbitals is six since there are three p orbitals.
From utedzz.blogspot.com
Periodic Table Groups Metals Periodic Table Timeline P Block Periodic Table Of Elements Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. They get their name from the fact that their valence electrons, or the electrons in the outermost shell, reside in the p orbital. Interact with other chemicals by losing, gaining, or sharing the valence electrons. The maximum number of electrons that can occur in a set of p orbitals. P Block Periodic Table Of Elements.
From insidechem.blogspot.com
Block periodic table INSIDE CHEMISTRY P Block Periodic Table Of Elements This block is situated at the extreme right of the periodic table. Interact with other chemicals by losing, gaining, or sharing the valence electrons. They include the last six element groups of the table (except for helium). They get their name from the fact that their valence electrons, or the electrons in the outermost shell, reside in the p orbital.. P Block Periodic Table Of Elements.
From bhentertainmentworld.blogspot.com
Periodic Table S And P Block Elements BHe P Block Periodic Table Of Elements The maximum number of electrons that can occur in a set of p orbitals is six since there are three p orbitals. Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. They include the last six element groups of the table (except for helium). This block is situated at the extreme right of the periodic table. Interact with. P Block Periodic Table Of Elements.
From lynceans.org
periodic table of elements The Lyncean Group of San Diego P Block Periodic Table Of Elements Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. Interact with other chemicals by losing, gaining, or sharing the valence electrons. They include the last six element groups of the table (except for helium). The maximum number of electrons that can occur in a set of p orbitals is six since there are three p orbitals. This block. P Block Periodic Table Of Elements.
From www.chemistrymadeeasy.com
Periodic table Chemistry Made Easy P Block Periodic Table Of Elements They get their name from the fact that their valence electrons, or the electrons in the outermost shell, reside in the p orbital. The “p” refers to the principal and azimuthal quantum number 1. Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. The maximum number of electrons that can occur in a set of p orbitals is. P Block Periodic Table Of Elements.
From testbook.com
Blocks of the Periodic Table sblock, pblock, dblock, fblock P Block Periodic Table Of Elements This block is situated at the extreme right of the periodic table. They include the last six element groups of the table (except for helium). The maximum number of electrons that can occur in a set of p orbitals is six since there are three p orbitals. The “p” refers to the principal and azimuthal quantum number 1. Include carbon,. P Block Periodic Table Of Elements.
From newtondesk.com
Block Classification of Periodic Table Elements Periods and Groups P Block Periodic Table Of Elements They get their name from the fact that their valence electrons, or the electrons in the outermost shell, reside in the p orbital. They include the last six element groups of the table (except for helium). The maximum number of electrons that can occur in a set of p orbitals is six since there are three p orbitals. This block. P Block Periodic Table Of Elements.
From www.chemistrylearner.com
pBlock Elements Chemistry Learner P Block Periodic Table Of Elements They get their name from the fact that their valence electrons, or the electrons in the outermost shell, reside in the p orbital. Interact with other chemicals by losing, gaining, or sharing the valence electrons. They include the last six element groups of the table (except for helium). The maximum number of electrons that can occur in a set of. P Block Periodic Table Of Elements.
From sciencenotes.org
Periodic Table Blocks of Elements P Block Periodic Table Of Elements Interact with other chemicals by losing, gaining, or sharing the valence electrons. Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. The maximum number of electrons that can occur in a set of p orbitals is six since there are three p orbitals. This block is situated at the extreme right of the periodic table. They include the. P Block Periodic Table Of Elements.
From chemavishkar.blogspot.com
PBLOCK ELEMENTS OF THE MODERN PERIODIC TABLE. What are P BLOCK P Block Periodic Table Of Elements They get their name from the fact that their valence electrons, or the electrons in the outermost shell, reside in the p orbital. This block is situated at the extreme right of the periodic table. Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. Interact with other chemicals by losing, gaining, or sharing the valence electrons. The maximum. P Block Periodic Table Of Elements.
From www.priyamstudycentre.com
Periodic Table Elements, Definition, Groups, Periods, Blocks P Block Periodic Table Of Elements They include the last six element groups of the table (except for helium). This block is situated at the extreme right of the periodic table. The maximum number of electrons that can occur in a set of p orbitals is six since there are three p orbitals. The “p” refers to the principal and azimuthal quantum number 1. They get. P Block Periodic Table Of Elements.
From pubchem.ncbi.nlm.nih.gov
Periodic Table of Elements PubChem P Block Periodic Table Of Elements The “p” refers to the principal and azimuthal quantum number 1. They include the last six element groups of the table (except for helium). They get their name from the fact that their valence electrons, or the electrons in the outermost shell, reside in the p orbital. Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. Interact with. P Block Periodic Table Of Elements.
From utedzz.blogspot.com
Periodic Table Blocks Labeled Periodic Table Timeline P Block Periodic Table Of Elements They include the last six element groups of the table (except for helium). This block is situated at the extreme right of the periodic table. Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. They get their name from the fact that their valence electrons, or the electrons in the outermost shell, reside in the p orbital. The. P Block Periodic Table Of Elements.
From www.alamy.com
Periodic table of the elements colored according to their block s, p P Block Periodic Table Of Elements Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. They include the last six element groups of the table (except for helium). Interact with other chemicals by losing, gaining, or sharing the valence electrons. This block is situated at the extreme right of the periodic table. They get their name from the fact that their valence electrons, or. P Block Periodic Table Of Elements.
From sciencenotes.org
Periodic Table Blocks of Elements P Block Periodic Table Of Elements Interact with other chemicals by losing, gaining, or sharing the valence electrons. The maximum number of electrons that can occur in a set of p orbitals is six since there are three p orbitals. They include the last six element groups of the table (except for helium). They get their name from the fact that their valence electrons, or the. P Block Periodic Table Of Elements.
From sciencenotes.org
S Block Elements and Their Properties P Block Periodic Table Of Elements The “p” refers to the principal and azimuthal quantum number 1. They get their name from the fact that their valence electrons, or the electrons in the outermost shell, reside in the p orbital. This block is situated at the extreme right of the periodic table. Interact with other chemicals by losing, gaining, or sharing the valence electrons. The maximum. P Block Periodic Table Of Elements.
From newtondesk.com
Block Classification of Periodic Table Elements Periods and Groups P Block Periodic Table Of Elements This block is situated at the extreme right of the periodic table. They get their name from the fact that their valence electrons, or the electrons in the outermost shell, reside in the p orbital. The maximum number of electrons that can occur in a set of p orbitals is six since there are three p orbitals. Include carbon, nitrogen,. P Block Periodic Table Of Elements.
From studigoo.com
P Block elements notes for Neet StudiGoo P Block Periodic Table Of Elements Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. Interact with other chemicals by losing, gaining, or sharing the valence electrons. The “p” refers to the principal and azimuthal quantum number 1. The maximum number of electrons that can occur in a set of p orbitals is six since there are three p orbitals. They include the last. P Block Periodic Table Of Elements.
From www.youtube.com
How To Find The Atomic Number Of S,P Block Elements In Periodic Table P Block Periodic Table Of Elements The maximum number of electrons that can occur in a set of p orbitals is six since there are three p orbitals. This block is situated at the extreme right of the periodic table. Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. They include the last six element groups of the table (except for helium). The “p”. P Block Periodic Table Of Elements.
From www.researchgate.net
1 pblock of the periodic table of the elements. B, C and N are in P Block Periodic Table Of Elements Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. The maximum number of electrons that can occur in a set of p orbitals is six since there are three p orbitals. Interact with other chemicals by losing, gaining, or sharing the valence electrons. This block is situated at the extreme right of the periodic table. The “p” refers. P Block Periodic Table Of Elements.
From www.animalia-life.club
Periodic Table Of Elements With Names And Symbols And Atomic Mass And P Block Periodic Table Of Elements Interact with other chemicals by losing, gaining, or sharing the valence electrons. Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. They include the last six element groups of the table (except for helium). The “p” refers to the principal and azimuthal quantum number 1. The maximum number of electrons that can occur in a set of p. P Block Periodic Table Of Elements.
From utedzz.blogspot.com
Periodic Table Blocks S P D F Periodic Table Timeline P Block Periodic Table Of Elements Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. The “p” refers to the principal and azimuthal quantum number 1. They get their name from the fact that their valence electrons, or the electrons in the outermost shell, reside in the p orbital. Interact with other chemicals by losing, gaining, or sharing the valence electrons. The maximum number. P Block Periodic Table Of Elements.
From www.ck12.org
The Periodic Table and Electron Configurations CK12 Foundation P Block Periodic Table Of Elements The maximum number of electrons that can occur in a set of p orbitals is six since there are three p orbitals. The “p” refers to the principal and azimuthal quantum number 1. They get their name from the fact that their valence electrons, or the electrons in the outermost shell, reside in the p orbital. Include carbon, nitrogen, oxygen,. P Block Periodic Table Of Elements.
From www.congress-intercultural.eu
P Block Elements Online Cheap www.congressintercultural.eu P Block Periodic Table Of Elements They include the last six element groups of the table (except for helium). This block is situated at the extreme right of the periodic table. Interact with other chemicals by losing, gaining, or sharing the valence electrons. The “p” refers to the principal and azimuthal quantum number 1. Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. They. P Block Periodic Table Of Elements.
From www.priyamstudycentre.com
f block Elements Lanthanides and Actinides Periodic Table P Block Periodic Table Of Elements Interact with other chemicals by losing, gaining, or sharing the valence electrons. They include the last six element groups of the table (except for helium). This block is situated at the extreme right of the periodic table. The maximum number of electrons that can occur in a set of p orbitals is six since there are three p orbitals. They. P Block Periodic Table Of Elements.
From www.slideserve.com
PPT The pblock elements PowerPoint Presentation ID649358 P Block Periodic Table Of Elements This block is situated at the extreme right of the periodic table. Interact with other chemicals by losing, gaining, or sharing the valence electrons. The “p” refers to the principal and azimuthal quantum number 1. Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. They include the last six element groups of the table (except for helium). The. P Block Periodic Table Of Elements.
From newtondesk.com
Block Classification of Periodic Table Elements Periods and Groups P Block Periodic Table Of Elements Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. Interact with other chemicals by losing, gaining, or sharing the valence electrons. They get their name from the fact that their valence electrons, or the electrons in the outermost shell, reside in the p orbital. This block is situated at the extreme right of the periodic table. The maximum. P Block Periodic Table Of Elements.
From animalia-life.club
Representative Elements Periodic Table P Block Periodic Table Of Elements They include the last six element groups of the table (except for helium). This block is situated at the extreme right of the periodic table. Interact with other chemicals by losing, gaining, or sharing the valence electrons. The “p” refers to the principal and azimuthal quantum number 1. They get their name from the fact that their valence electrons, or. P Block Periodic Table Of Elements.
From chemistnotes.com
d block elements, name list, useful properties Chemistry Notes P Block Periodic Table Of Elements This block is situated at the extreme right of the periodic table. They get their name from the fact that their valence electrons, or the electrons in the outermost shell, reside in the p orbital. Interact with other chemicals by losing, gaining, or sharing the valence electrons. The “p” refers to the principal and azimuthal quantum number 1. Include carbon,. P Block Periodic Table Of Elements.
From everythinghere007.blogspot.com
Everything's Here sblock Elements and pblock Elements P Block Periodic Table Of Elements They get their name from the fact that their valence electrons, or the electrons in the outermost shell, reside in the p orbital. The “p” refers to the principal and azimuthal quantum number 1. They include the last six element groups of the table (except for helium). Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. The maximum. P Block Periodic Table Of Elements.
From homedeso.vercel.app
Periodic Table Blocks Of Elements P Block Periodic Table Of Elements The “p” refers to the principal and azimuthal quantum number 1. Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. The maximum number of electrons that can occur in a set of p orbitals is six since there are three p orbitals. They get their name from the fact that their valence electrons, or the electrons in the. P Block Periodic Table Of Elements.
From blog.askiitians.com
These Daily Uses of pblock Elements will Surprise You askIITians P Block Periodic Table Of Elements They include the last six element groups of the table (except for helium). The “p” refers to the principal and azimuthal quantum number 1. They get their name from the fact that their valence electrons, or the electrons in the outermost shell, reside in the p orbital. This block is situated at the extreme right of the periodic table. The. P Block Periodic Table Of Elements.
From www.britannica.com
periodic table Definition & Groups Britannica P Block Periodic Table Of Elements They get their name from the fact that their valence electrons, or the electrons in the outermost shell, reside in the p orbital. The maximum number of electrons that can occur in a set of p orbitals is six since there are three p orbitals. This block is situated at the extreme right of the periodic table. They include the. P Block Periodic Table Of Elements.
From www.slideserve.com
PPT The pblock elements PowerPoint Presentation ID649358 P Block Periodic Table Of Elements Interact with other chemicals by losing, gaining, or sharing the valence electrons. Include carbon, nitrogen, oxygen, sulfur, halogens, and many other common elements. This block is situated at the extreme right of the periodic table. They get their name from the fact that their valence electrons, or the electrons in the outermost shell, reside in the p orbital. The maximum. P Block Periodic Table Of Elements.
From mavink.com
Periodic Table Split Into Blocks P Block Periodic Table Of Elements The “p” refers to the principal and azimuthal quantum number 1. The maximum number of electrons that can occur in a set of p orbitals is six since there are three p orbitals. Interact with other chemicals by losing, gaining, or sharing the valence electrons. They include the last six element groups of the table (except for helium). This block. P Block Periodic Table Of Elements.