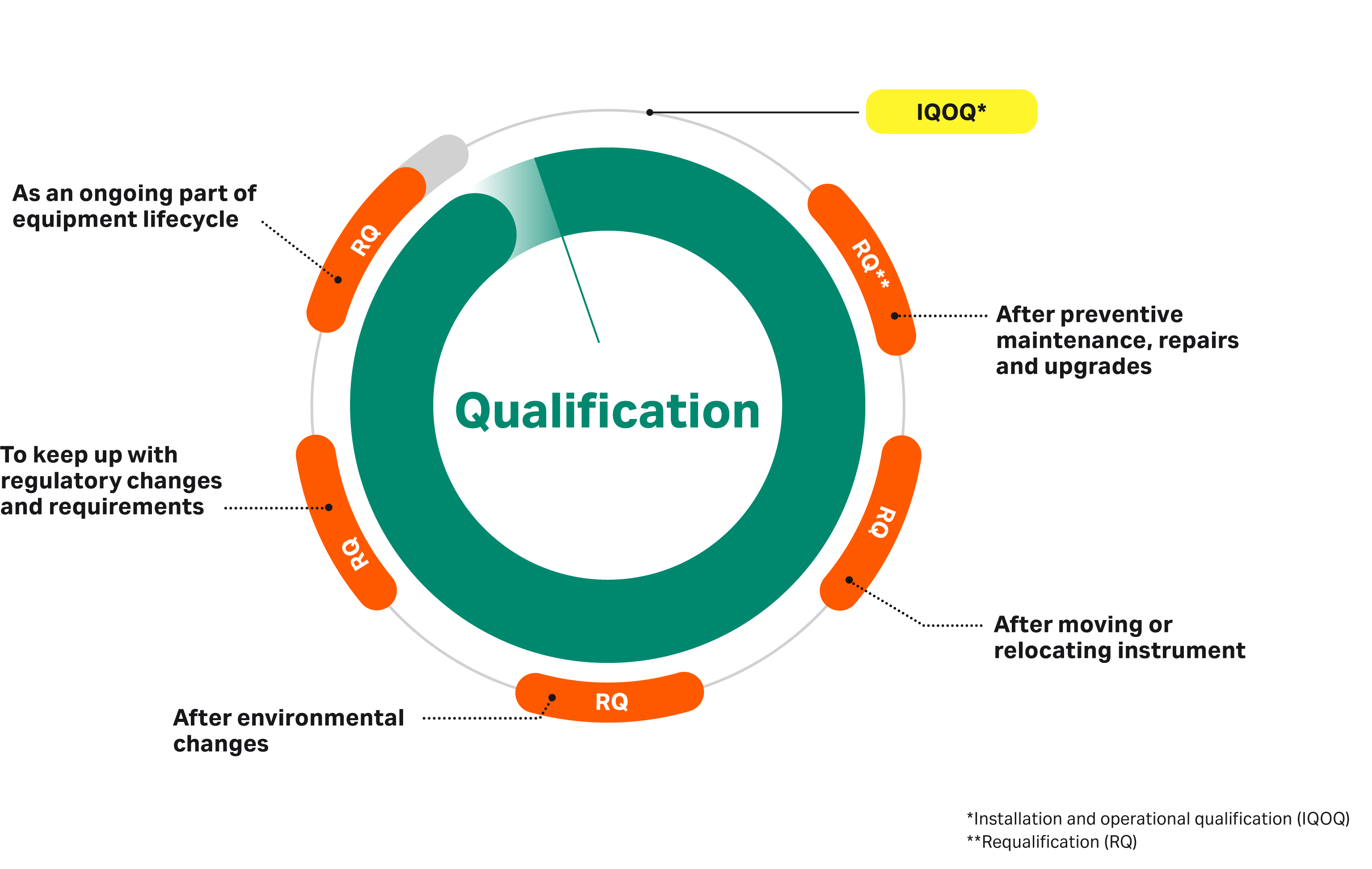
Utility Qualification Guidelines . In addition to the main text, appendices on some validation and. 3.1 the purpose of this document is to provide guidance for gmp inspectors to use for training purposes and in preparation for inspections. This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. It is important and mandatory to validate the utilities before its use in pharmaceutical manufacturing. 1.2 these guidelines cover the general principles of qualification and validation. Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. The overarching text presented in this annex constitutes the general principles of the new guidance on validation.
from www.cytivalifesciences.com.cn
In addition to the main text, appendices on some validation and. The overarching text presented in this annex constitutes the general principles of the new guidance on validation. Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. It is important and mandatory to validate the utilities before its use in pharmaceutical manufacturing. 3.1 the purpose of this document is to provide guidance for gmp inspectors to use for training purposes and in preparation for inspections. 1.2 these guidelines cover the general principles of qualification and validation. This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and.
Stay compliant instrument qualification for cGMP Cytiva
Utility Qualification Guidelines Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. 1.2 these guidelines cover the general principles of qualification and validation. It is important and mandatory to validate the utilities before its use in pharmaceutical manufacturing. 3.1 the purpose of this document is to provide guidance for gmp inspectors to use for training purposes and in preparation for inspections. In addition to the main text, appendices on some validation and. The overarching text presented in this annex constitutes the general principles of the new guidance on validation. Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and.
From medvacon.com
Facilities and Utilities Commissioning & Qualification — Medvacon Life Utility Qualification Guidelines 3.1 the purpose of this document is to provide guidance for gmp inspectors to use for training purposes and in preparation for inspections. Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique. Utility Qualification Guidelines.
From www.calameo.com
Calaméo Utility Mapping Training For Proqual Level 3 Qualification Utility Qualification Guidelines Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. In addition to the main text, appendices on some validation and. 1.2 these guidelines cover the general principles of qualification and validation. This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to. Utility Qualification Guidelines.
From www.scribd.com
Criticial Utility Qualification Part1 PDF Verification And Utility Qualification Guidelines This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. 3.1 the purpose of this document is to provide guidance for gmp inspectors to use for training purposes and in preparation for inspections. In addition to the main text, appendices on some validation. Utility Qualification Guidelines.
From www.youtube.com
Image Answer UtilityEnglish Qualification uhrs Ali Tips YouTube Utility Qualification Guidelines The overarching text presented in this annex constitutes the general principles of the new guidance on validation. It is important and mandatory to validate the utilities before its use in pharmaceutical manufacturing. In addition to the main text, appendices on some validation and. This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique. Utility Qualification Guidelines.
From dokumen.tips
(PDF) Procedures & Guidelines for Contractor PreQualification Utility Qualification Guidelines It is important and mandatory to validate the utilities before its use in pharmaceutical manufacturing. 3.1 the purpose of this document is to provide guidance for gmp inspectors to use for training purposes and in preparation for inspections. The overarching text presented in this annex constitutes the general principles of the new guidance on validation. Installation qualification (iq) provides documented. Utility Qualification Guidelines.
From www.scribd.com
Critical Utilities Qualification PDF Verification And Validation Utility Qualification Guidelines The overarching text presented in this annex constitutes the general principles of the new guidance on validation. It is important and mandatory to validate the utilities before its use in pharmaceutical manufacturing. In addition to the main text, appendices on some validation and. Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their. Utility Qualification Guidelines.
From studylib.net
Guidelines validation qualification systemsutilitiesequipment QAS16 Utility Qualification Guidelines Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. It is important and mandatory to validate the utilities before its use in pharmaceutical manufacturing. The overarching text presented in this annex constitutes the general principles of the new guidance on validation. 1.2 these guidelines cover the general principles of. Utility Qualification Guidelines.
From www.scribd.com
Effective Qualification of Critical Utilities PDF PDF Verification Utility Qualification Guidelines Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. 1.2 these guidelines cover the general principles of qualification and validation. 3.1 the purpose of this document is to provide guidance for gmp inspectors to use for training purposes and in preparation for inspections. This guide covers water systems, pure. Utility Qualification Guidelines.
From pharmablog.in
Qualification of Equipment / Instrument / Utilities SOP PharmaBlog Utility Qualification Guidelines In addition to the main text, appendices on some validation and. Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. 3.1 the purpose of this document is to provide guidance for gmp inspectors to use for training purposes and in preparation for inspections. This guide covers water systems, pure. Utility Qualification Guidelines.
From www.zoko.io
Guidelines for Utility and Marketing Templates Utility Qualification Guidelines Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. It is important and mandatory to validate the utilities before its use in pharmaceutical manufacturing. In addition to the main text, appendices on some validation and. The overarching text presented in this annex constitutes the general principles of the new. Utility Qualification Guidelines.
From www.yuncomed.com
Utility Model Qualification Certificate Kunshan Yunco Precision Co Utility Qualification Guidelines 1.2 these guidelines cover the general principles of qualification and validation. Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. 3.1 the purpose of this document is to provide guidance for gmp inspectors to use for training purposes and in preparation for inspections. In addition to the main text,. Utility Qualification Guidelines.
From www.slideserve.com
PPT Utility Analysis and the Impact of In re Fisher PowerPoint Utility Qualification Guidelines Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. In addition to the main text, appendices on some validation and. It is important and mandatory to validate the utilities before its use in pharmaceutical manufacturing. 3.1 the purpose of this document is to provide guidance for gmp inspectors to. Utility Qualification Guidelines.
From www.scribd.com
Utilities Qualification PDF Water Purification Verification And Utility Qualification Guidelines Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. 1.2 these guidelines cover the general principles of qualification and validation. 3.1 the purpose of this document is to provide guidance for gmp inspectors to use for training purposes and in preparation for inspections. The overarching text presented in this. Utility Qualification Guidelines.
From www.getreskilled.com
Qualification vs Validation in Pharma GetReskilled Utility Qualification Guidelines The overarching text presented in this annex constitutes the general principles of the new guidance on validation. This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. 1.2 these guidelines cover the general principles of qualification and validation. Installation qualification (iq) provides documented. Utility Qualification Guidelines.
From www.scribd.com
VAL095FacilityandUtilityValidationGuidelinesample PDF Utility Qualification Guidelines It is important and mandatory to validate the utilities before its use in pharmaceutical manufacturing. This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. 3.1 the purpose of this document is to provide guidance for gmp inspectors to use for training purposes. Utility Qualification Guidelines.
From www.cytivalifesciences.com.cn
Stay compliant instrument qualification for cGMP Cytiva Utility Qualification Guidelines Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. In addition to the main text, appendices on some validation and. This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. The overarching. Utility Qualification Guidelines.
From pharmablog.in
Qualification of Equipment / Instrument / Utilities SOP PharmaBlog Utility Qualification Guidelines The overarching text presented in this annex constitutes the general principles of the new guidance on validation. Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining. Utility Qualification Guidelines.
From www.scribd.com
HVAC, Water, and Other Critical Utility Qualifications PDF Utility Qualification Guidelines This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. 3.1 the purpose of this document is to provide guidance for gmp inspectors to use for training purposes and in preparation for inspections. Installation qualification (iq) provides documented evidence that the facilities and. Utility Qualification Guidelines.
From pharmaguidances.com
Qualification Of Existing Facilities Utility Qualification Guidelines 1.2 these guidelines cover the general principles of qualification and validation. The overarching text presented in this annex constitutes the general principles of the new guidance on validation. This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. In addition to the main. Utility Qualification Guidelines.
From assets.velvetjobs.com
Utility Worker Job Description Velvet Jobs Utility Qualification Guidelines This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. 1.2 these guidelines cover the general principles of qualification and validation. It is important and mandatory to validate the utilities before its use in pharmaceutical manufacturing. The overarching text presented in this annex. Utility Qualification Guidelines.
From www.youtube.com
Installation Qualification Format Checklist YouTube Utility Qualification Guidelines This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. 3.1 the purpose of this document is to provide guidance for gmp inspectors to use for training purposes and in preparation for inspections. It is important and mandatory to validate the utilities before. Utility Qualification Guidelines.
From www.slideserve.com
PPT VALIDATION METHODOLOGY PowerPoint Presentation, free download Utility Qualification Guidelines In addition to the main text, appendices on some validation and. The overarching text presented in this annex constitutes the general principles of the new guidance on validation. Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. 3.1 the purpose of this document is to provide guidance for gmp. Utility Qualification Guidelines.
From gggi.org
Regulatory Guidelines on the Procurement of UtilityScale Renewable Utility Qualification Guidelines It is important and mandatory to validate the utilities before its use in pharmaceutical manufacturing. The overarching text presented in this annex constitutes the general principles of the new guidance on validation. 3.1 the purpose of this document is to provide guidance for gmp inspectors to use for training purposes and in preparation for inspections. This guide covers water systems,. Utility Qualification Guidelines.
From www.slideserve.com
PPT LRI Validation Suite Meeting PowerPoint Presentation, free Utility Qualification Guidelines 3.1 the purpose of this document is to provide guidance for gmp inspectors to use for training purposes and in preparation for inspections. 1.2 these guidelines cover the general principles of qualification and validation. It is important and mandatory to validate the utilities before its use in pharmaceutical manufacturing. This guide covers water systems, pure steam, compressed air, and medical. Utility Qualification Guidelines.
From slidetodoc.com
Basic Principles of GMP Qualification and Validation Section Utility Qualification Guidelines This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. 3.1 the purpose of this document is to provide guidance for gmp inspectors to use for training purposes and in preparation for inspections. 1.2 these guidelines cover the general principles of qualification and. Utility Qualification Guidelines.
From www.pinterest.com
Qualification Stages For Equipment, Facilities, Utilities And System Utility Qualification Guidelines 1.2 these guidelines cover the general principles of qualification and validation. Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. The overarching text presented in this annex constitutes the general principles of the new guidance on validation. This guide covers water systems, pure steam, compressed air, and medical gases,. Utility Qualification Guidelines.
From www.scribd.com
Critical Utility Qualification PDF Hvac Verification And Validation Utility Qualification Guidelines Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. The overarching text presented in this annex constitutes the general principles of the new guidance on validation. This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining. Utility Qualification Guidelines.
From www.ncver.edu.au
Uptake and utility of VET qualifications infographic Utility Qualification Guidelines 1.2 these guidelines cover the general principles of qualification and validation. In addition to the main text, appendices on some validation and. Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to. Utility Qualification Guidelines.
From www.presentationeze.com
Equipment Validation Facility Qualification Material Utility Qualification Guidelines 1.2 these guidelines cover the general principles of qualification and validation. In addition to the main text, appendices on some validation and. Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. It is important and mandatory to validate the utilities before its use in pharmaceutical manufacturing. The overarching text. Utility Qualification Guidelines.
From www.scribd.com
Content Utility Rating Guidelines v2.0 PDF Multi Level Marketing Utility Qualification Guidelines In addition to the main text, appendices on some validation and. It is important and mandatory to validate the utilities before its use in pharmaceutical manufacturing. The overarching text presented in this annex constitutes the general principles of the new guidance on validation. 1.2 these guidelines cover the general principles of qualification and validation. 3.1 the purpose of this document. Utility Qualification Guidelines.
From www.scribd.com
Pharmaceutical Utility Qualification PDF Verification And Utility Qualification Guidelines Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. It is important and mandatory to validate the utilities before its use in pharmaceutical manufacturing. This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout. Utility Qualification Guidelines.
From dokumen.tips
(PDF) Level 3 Certificate in Utility Mapping and Surveying Utility Qualification Guidelines Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. It is important and mandatory to validate the utilities before its use in pharmaceutical manufacturing. The overarching text presented in this annex constitutes the general principles of the new guidance on validation. 1.2 these guidelines cover the general principles of. Utility Qualification Guidelines.
From www.scribd.com
Guidance on Qualification of existing facilities, systems, equipment Utility Qualification Guidelines It is important and mandatory to validate the utilities before its use in pharmaceutical manufacturing. This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. 3.1 the purpose of this document is to provide guidance for gmp inspectors to use for training purposes. Utility Qualification Guidelines.
From www.eurofins.it
Facility, utilities and Equipment Qualification Eurofins Italia Utility Qualification Guidelines In addition to the main text, appendices on some validation and. This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. It is important and mandatory to validate the utilities before its use in pharmaceutical manufacturing. The overarching text presented in this annex. Utility Qualification Guidelines.
From www.energy.gov
Federal Solar Tax Credits for Businesses Department of Energy Utility Qualification Guidelines This guide covers water systems, pure steam, compressed air, and medical gases, as well as processes unique to critical utilities, such as maintaining piping layout drawings and. In addition to the main text, appendices on some validation and. Installation qualification (iq) provides documented evidence that the facilities and utilities have been installed according to their design specifications, user. The overarching. Utility Qualification Guidelines.