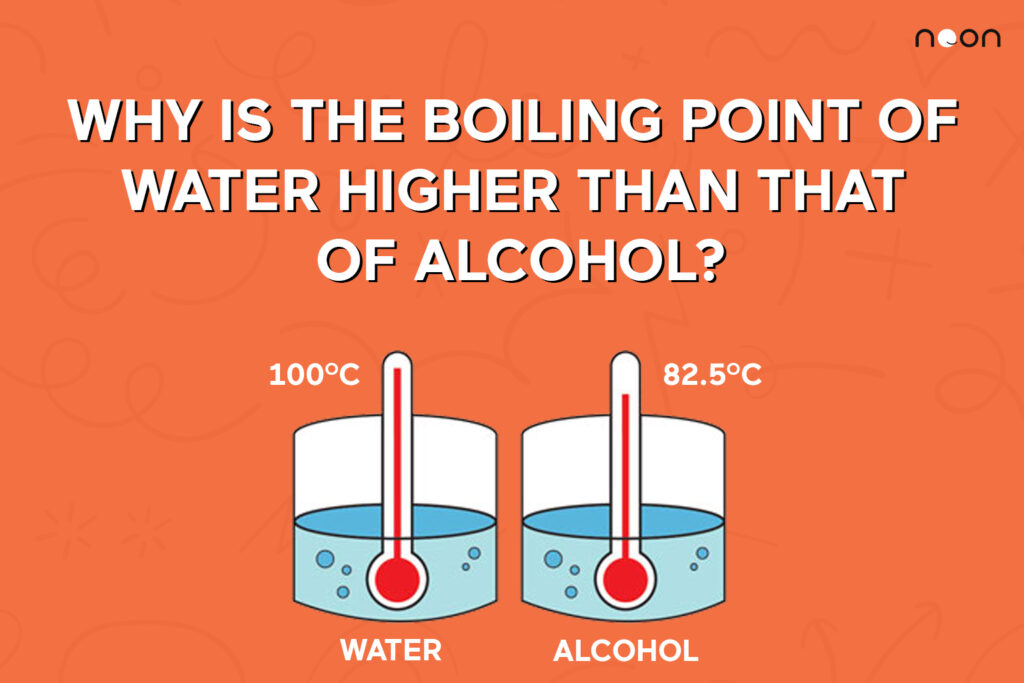
Why Is Water S Boiling Point Higher Than Methane . Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. Why does water have a higher boiling point than methane? The stronger the intermolecular forces, the higher the boiling point. Ethanol (c2h5oh) has a higher boiling point than methane (ch4) because it has stronger intermolecular forces, specifically. Water has a higher boiling point because the hydrogen bonds that form among water molecules are stronger than the van der waals. Water has a higher boiling point than methane because it forms strong hydrogen bonds, whereas option d) methane only has. The reason water has a significantly much higher boiling point than methane is that water molecules have hydrogen bonds between them. Methane has van der waal's forces between molecules whereas water has.
from www.learnatnoon.com
Methane has van der waal's forces between molecules whereas water has. Ethanol (c2h5oh) has a higher boiling point than methane (ch4) because it has stronger intermolecular forces, specifically. Water has a higher boiling point than methane because it forms strong hydrogen bonds, whereas option d) methane only has. Why does water have a higher boiling point than methane? Water has a higher boiling point because the hydrogen bonds that form among water molecules are stronger than the van der waals. The reason water has a significantly much higher boiling point than methane is that water molecules have hydrogen bonds between them. Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. The stronger the intermolecular forces, the higher the boiling point.
The boiling point of water and alcohol explained Noon Academy
Why Is Water S Boiling Point Higher Than Methane The reason water has a significantly much higher boiling point than methane is that water molecules have hydrogen bonds between them. Water has a higher boiling point because the hydrogen bonds that form among water molecules are stronger than the van der waals. Ethanol (c2h5oh) has a higher boiling point than methane (ch4) because it has stronger intermolecular forces, specifically. The stronger the intermolecular forces, the higher the boiling point. Water has a higher boiling point than methane because it forms strong hydrogen bonds, whereas option d) methane only has. The reason water has a significantly much higher boiling point than methane is that water molecules have hydrogen bonds between them. Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. Methane has van der waal's forces between molecules whereas water has. Why does water have a higher boiling point than methane?
From www.toppr.com
H2O has higher boiling point than H2S due to Why Is Water S Boiling Point Higher Than Methane The stronger the intermolecular forces, the higher the boiling point. Why does water have a higher boiling point than methane? Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. The reason water has a significantly much higher boiling point than methane is that water molecules have hydrogen bonds between them. Methane has van der. Why Is Water S Boiling Point Higher Than Methane.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Why Is Water S Boiling Point Higher Than Methane The reason water has a significantly much higher boiling point than methane is that water molecules have hydrogen bonds between them. Water has a higher boiling point than methane because it forms strong hydrogen bonds, whereas option d) methane only has. Methane has van der waal's forces between molecules whereas water has. Why does water have a higher boiling point. Why Is Water S Boiling Point Higher Than Methane.
From www.youtube.com
Comparing Water and Methane (2016) IB Biology YouTube Why Is Water S Boiling Point Higher Than Methane The reason water has a significantly much higher boiling point than methane is that water molecules have hydrogen bonds between them. The stronger the intermolecular forces, the higher the boiling point. Water has a higher boiling point because the hydrogen bonds that form among water molecules are stronger than the van der waals. Water has a higher boiling point than. Why Is Water S Boiling Point Higher Than Methane.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts Why Is Water S Boiling Point Higher Than Methane Ethanol (c2h5oh) has a higher boiling point than methane (ch4) because it has stronger intermolecular forces, specifically. The stronger the intermolecular forces, the higher the boiling point. Water has a higher boiling point because the hydrogen bonds that form among water molecules are stronger than the van der waals. Why does water have a higher boiling point than methane? Two. Why Is Water S Boiling Point Higher Than Methane.
From mavink.com
Water Boiling Point Pressure Chart Why Is Water S Boiling Point Higher Than Methane Water has a higher boiling point because the hydrogen bonds that form among water molecules are stronger than the van der waals. Ethanol (c2h5oh) has a higher boiling point than methane (ch4) because it has stronger intermolecular forces, specifically. Water has a higher boiling point than methane because it forms strong hydrogen bonds, whereas option d) methane only has. Why. Why Is Water S Boiling Point Higher Than Methane.
From engineeringstuff.co.in
What is Boiling point Engineeringstuff Why Is Water S Boiling Point Higher Than Methane Ethanol (c2h5oh) has a higher boiling point than methane (ch4) because it has stronger intermolecular forces, specifically. Methane has van der waal's forces between molecules whereas water has. Water has a higher boiling point because the hydrogen bonds that form among water molecules are stronger than the van der waals. The reason water has a significantly much higher boiling point. Why Is Water S Boiling Point Higher Than Methane.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills Why Is Water S Boiling Point Higher Than Methane Why does water have a higher boiling point than methane? Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. Ethanol (c2h5oh) has a higher boiling point than methane (ch4) because it has stronger intermolecular forces, specifically. Water has a higher boiling point than methane because it forms strong hydrogen bonds, whereas option d) methane. Why Is Water S Boiling Point Higher Than Methane.
From brainly.ph
1.Water(H20) has higher boiling point and melting point than methane Why Is Water S Boiling Point Higher Than Methane The reason water has a significantly much higher boiling point than methane is that water molecules have hydrogen bonds between them. Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. Water has a higher boiling point because the hydrogen bonds that form among water molecules are stronger than the van der waals. Water has. Why Is Water S Boiling Point Higher Than Methane.
From exyaeabov.blob.core.windows.net
What Is The Boiling Point Melting Point And Freezing Point Of Water at Why Is Water S Boiling Point Higher Than Methane Why does water have a higher boiling point than methane? Water has a higher boiling point because the hydrogen bonds that form among water molecules are stronger than the van der waals. Water has a higher boiling point than methane because it forms strong hydrogen bonds, whereas option d) methane only has. Methane has van der waal's forces between molecules. Why Is Water S Boiling Point Higher Than Methane.
From www.youtube.com
Which Compound Has a Higher Boiling Point? Intermolecular Force Boiling Why Is Water S Boiling Point Higher Than Methane Ethanol (c2h5oh) has a higher boiling point than methane (ch4) because it has stronger intermolecular forces, specifically. Why does water have a higher boiling point than methane? Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. The reason water has a significantly much higher boiling point than methane is that water molecules have hydrogen. Why Is Water S Boiling Point Higher Than Methane.
From www.myopencountry.com
Boiling Water at Higher Altitude What You Need to Know My Open Country Why Is Water S Boiling Point Higher Than Methane Why does water have a higher boiling point than methane? Ethanol (c2h5oh) has a higher boiling point than methane (ch4) because it has stronger intermolecular forces, specifically. Water has a higher boiling point because the hydrogen bonds that form among water molecules are stronger than the van der waals. The stronger the intermolecular forces, the higher the boiling point. The. Why Is Water S Boiling Point Higher Than Methane.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Why Is Water S Boiling Point Higher Than Methane Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. Water has a higher boiling point than methane because it forms strong hydrogen bonds, whereas option d) methane only has. Methane has van der waal's forces between molecules whereas water has. Why does water have a higher boiling point than methane? Ethanol (c2h5oh) has a. Why Is Water S Boiling Point Higher Than Methane.
From www.studyxapp.com
the boiling points alkanes boiling molecular point formula name c Why Is Water S Boiling Point Higher Than Methane Ethanol (c2h5oh) has a higher boiling point than methane (ch4) because it has stronger intermolecular forces, specifically. Why does water have a higher boiling point than methane? The reason water has a significantly much higher boiling point than methane is that water molecules have hydrogen bonds between them. Water has a higher boiling point because the hydrogen bonds that form. Why Is Water S Boiling Point Higher Than Methane.
From dxojohlhr.blob.core.windows.net
Is Water Boiling Points at Lillian Bergeron blog Why Is Water S Boiling Point Higher Than Methane Methane has van der waal's forces between molecules whereas water has. Why does water have a higher boiling point than methane? Water has a higher boiling point than methane because it forms strong hydrogen bonds, whereas option d) methane only has. Ethanol (c2h5oh) has a higher boiling point than methane (ch4) because it has stronger intermolecular forces, specifically. Two oxygen. Why Is Water S Boiling Point Higher Than Methane.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Why Is Water S Boiling Point Higher Than Methane The stronger the intermolecular forces, the higher the boiling point. Methane has van der waal's forces between molecules whereas water has. The reason water has a significantly much higher boiling point than methane is that water molecules have hydrogen bonds between them. Ethanol (c2h5oh) has a higher boiling point than methane (ch4) because it has stronger intermolecular forces, specifically. Two. Why Is Water S Boiling Point Higher Than Methane.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples Why Is Water S Boiling Point Higher Than Methane Water has a higher boiling point than methane because it forms strong hydrogen bonds, whereas option d) methane only has. Methane has van der waal's forces between molecules whereas water has. The stronger the intermolecular forces, the higher the boiling point. Why does water have a higher boiling point than methane? The reason water has a significantly much higher boiling. Why Is Water S Boiling Point Higher Than Methane.
From www.youtube.com
Why is the Boiling Point of water (H2O) so high? YouTube Why Is Water S Boiling Point Higher Than Methane The reason water has a significantly much higher boiling point than methane is that water molecules have hydrogen bonds between them. Methane has van der waal's forces between molecules whereas water has. Water has a higher boiling point than methane because it forms strong hydrogen bonds, whereas option d) methane only has. Two oxygen molecules are attracted to each other. Why Is Water S Boiling Point Higher Than Methane.
From klaubgmaz.blob.core.windows.net
What Is Boiling Point In Science at Lan Johnson blog Why Is Water S Boiling Point Higher Than Methane Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. Water has a higher boiling point than methane because it forms strong hydrogen bonds, whereas option d) methane only has. The reason water has a significantly much higher boiling point than methane is that water molecules have hydrogen bonds between them. The stronger the intermolecular. Why Is Water S Boiling Point Higher Than Methane.
From slideplayer.com
Gases Chapter ppt download Why Is Water S Boiling Point Higher Than Methane The stronger the intermolecular forces, the higher the boiling point. Water has a higher boiling point because the hydrogen bonds that form among water molecules are stronger than the van der waals. Water has a higher boiling point than methane because it forms strong hydrogen bonds, whereas option d) methane only has. The reason water has a significantly much higher. Why Is Water S Boiling Point Higher Than Methane.
From www.slideserve.com
PPT Water as a Polar Molecule PowerPoint Presentation, free download Why Is Water S Boiling Point Higher Than Methane The reason water has a significantly much higher boiling point than methane is that water molecules have hydrogen bonds between them. Why does water have a higher boiling point than methane? Water has a higher boiling point because the hydrogen bonds that form among water molecules are stronger than the van der waals. Methane has van der waal's forces between. Why Is Water S Boiling Point Higher Than Methane.
From www.numerade.com
SOLVED Why does water have a much higher boiling point than methane Why Is Water S Boiling Point Higher Than Methane Water has a higher boiling point because the hydrogen bonds that form among water molecules are stronger than the van der waals. Ethanol (c2h5oh) has a higher boiling point than methane (ch4) because it has stronger intermolecular forces, specifically. The stronger the intermolecular forces, the higher the boiling point. Why does water have a higher boiling point than methane? Water. Why Is Water S Boiling Point Higher Than Methane.
From slideplayer.com
Plants absorb water through their roots from soil or from a solution Why Is Water S Boiling Point Higher Than Methane The stronger the intermolecular forces, the higher the boiling point. Water has a higher boiling point because the hydrogen bonds that form among water molecules are stronger than the van der waals. Ethanol (c2h5oh) has a higher boiling point than methane (ch4) because it has stronger intermolecular forces, specifically. The reason water has a significantly much higher boiling point than. Why Is Water S Boiling Point Higher Than Methane.
From www.numerade.com
SOLVED'water has the highest and methane the lowest boiling point in Why Is Water S Boiling Point Higher Than Methane Water has a higher boiling point because the hydrogen bonds that form among water molecules are stronger than the van der waals. The reason water has a significantly much higher boiling point than methane is that water molecules have hydrogen bonds between them. Water has a higher boiling point than methane because it forms strong hydrogen bonds, whereas option d). Why Is Water S Boiling Point Higher Than Methane.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Why Is Water S Boiling Point Higher Than Methane Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. The stronger the intermolecular forces, the higher the boiling point. Water has a higher boiling point than methane because it forms strong hydrogen bonds, whereas option d) methane only has. Methane has van der waal's forces between molecules whereas water has. Water has a higher. Why Is Water S Boiling Point Higher Than Methane.
From www.numerade.com
SOLVEDUse the fact that water is a polar molecule to explain why water Why Is Water S Boiling Point Higher Than Methane The stronger the intermolecular forces, the higher the boiling point. Water has a higher boiling point than methane because it forms strong hydrogen bonds, whereas option d) methane only has. Ethanol (c2h5oh) has a higher boiling point than methane (ch4) because it has stronger intermolecular forces, specifically. Water has a higher boiling point because the hydrogen bonds that form among. Why Is Water S Boiling Point Higher Than Methane.
From pubchem.ncbi.nlm.nih.gov
Boiling Point Periodic Table of Elements PubChem Why Is Water S Boiling Point Higher Than Methane Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. The stronger the intermolecular forces, the higher the boiling point. Methane has van der waal's forces between molecules whereas water has. Why does water have a higher boiling point than methane? Ethanol (c2h5oh) has a higher boiling point than methane (ch4) because it has stronger. Why Is Water S Boiling Point Higher Than Methane.
From www.youtube.com
Difference in Boiling Points for H2O and H2S YouTube Why Is Water S Boiling Point Higher Than Methane Methane has van der waal's forces between molecules whereas water has. The reason water has a significantly much higher boiling point than methane is that water molecules have hydrogen bonds between them. Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. Water has a higher boiling point because the hydrogen bonds that form among. Why Is Water S Boiling Point Higher Than Methane.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Why Is Water S Boiling Point Higher Than Methane Water has a higher boiling point because the hydrogen bonds that form among water molecules are stronger than the van der waals. Methane has van der waal's forces between molecules whereas water has. Water has a higher boiling point than methane because it forms strong hydrogen bonds, whereas option d) methane only has. Ethanol (c2h5oh) has a higher boiling point. Why Is Water S Boiling Point Higher Than Methane.
From joixgvqec.blob.core.windows.net
Why Is The Boiling Point Of Water High at Tina Edmonds blog Why Is Water S Boiling Point Higher Than Methane The stronger the intermolecular forces, the higher the boiling point. The reason water has a significantly much higher boiling point than methane is that water molecules have hydrogen bonds between them. Ethanol (c2h5oh) has a higher boiling point than methane (ch4) because it has stronger intermolecular forces, specifically. Two oxygen molecules are attracted to each other through london dispersion forces. Why Is Water S Boiling Point Higher Than Methane.
From dxojohlhr.blob.core.windows.net
Is Water Boiling Points at Lillian Bergeron blog Why Is Water S Boiling Point Higher Than Methane Water has a higher boiling point because the hydrogen bonds that form among water molecules are stronger than the van der waals. Water has a higher boiling point than methane because it forms strong hydrogen bonds, whereas option d) methane only has. The stronger the intermolecular forces, the higher the boiling point. Ethanol (c2h5oh) has a higher boiling point than. Why Is Water S Boiling Point Higher Than Methane.
From slideplayer.com
Water and Aqueous Systems ppt download Why Is Water S Boiling Point Higher Than Methane Why does water have a higher boiling point than methane? Methane has van der waal's forces between molecules whereas water has. Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. The reason water has a significantly much higher boiling point than methane is that water molecules have hydrogen bonds between them. Ethanol (c2h5oh) has. Why Is Water S Boiling Point Higher Than Methane.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox Why Is Water S Boiling Point Higher Than Methane Water has a higher boiling point because the hydrogen bonds that form among water molecules are stronger than the van der waals. Why does water have a higher boiling point than methane? The stronger the intermolecular forces, the higher the boiling point. The reason water has a significantly much higher boiling point than methane is that water molecules have hydrogen. Why Is Water S Boiling Point Higher Than Methane.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy Why Is Water S Boiling Point Higher Than Methane Methane has van der waal's forces between molecules whereas water has. Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. Ethanol (c2h5oh) has a higher boiling point than methane (ch4) because it has stronger intermolecular forces, specifically. Water has a higher boiling point than methane because it forms strong hydrogen bonds, whereas option d). Why Is Water S Boiling Point Higher Than Methane.
From chemistnotes.com
Elevation of Boiling Point Definition/Equation/Molal Boiling Point Why Is Water S Boiling Point Higher Than Methane The stronger the intermolecular forces, the higher the boiling point. Water has a higher boiling point because the hydrogen bonds that form among water molecules are stronger than the van der waals. Methane has van der waal's forces between molecules whereas water has. Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. Ethanol (c2h5oh). Why Is Water S Boiling Point Higher Than Methane.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy Why Is Water S Boiling Point Higher Than Methane The reason water has a significantly much higher boiling point than methane is that water molecules have hydrogen bonds between them. Water has a higher boiling point because the hydrogen bonds that form among water molecules are stronger than the van der waals. Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. Water has. Why Is Water S Boiling Point Higher Than Methane.