Standard Enthalpy Of Formation Table . Learn the definition, equation and examples of standard enthalpy of formation, the energy change when one mole of a substance is formed from. The table covers alkanes, miscellaneous. Learn how to calculate or predict enthalpy changes for chemical reactions using standard enthalpy of formation, which is the enthalpy change when 1 mole of a pure substance forms from its elements. See examples, exercises, and a table of standard enthalpies of formation for common substances. In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in. Find the standard enthalpy of formation, gibbs free energy of formation, entropy and heat capacity of 370 inorganic compounds at 25°c. Find heat of formation values for various compounds and elements in kcal/mol or kj/mol. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Find the molar heat of formation (δh f) of various compounds at 25 degrees celsius and one atom from elements in their stable form. Learn how to calculate enthalpy changes using standard enthalpies of formation, which are tabulated for many compounds under standard conditions. Learn how to use this table for enthalpy calculations and the points to remember for the process. See examples, characteristics, and a table of standard enthalpy of formation values for common substances.
from
This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in. See examples, exercises, and a table of standard enthalpies of formation for common substances. Find the molar heat of formation (δh f) of various compounds at 25 degrees celsius and one atom from elements in their stable form. See examples, characteristics, and a table of standard enthalpy of formation values for common substances. Learn how to use this table for enthalpy calculations and the points to remember for the process. Learn how to calculate enthalpy changes using standard enthalpies of formation, which are tabulated for many compounds under standard conditions. The table covers alkanes, miscellaneous. Find the standard enthalpy of formation, gibbs free energy of formation, entropy and heat capacity of 370 inorganic compounds at 25°c. Learn how to calculate or predict enthalpy changes for chemical reactions using standard enthalpy of formation, which is the enthalpy change when 1 mole of a pure substance forms from its elements.
Standard Enthalpy Of Formation Table See examples, exercises, and a table of standard enthalpies of formation for common substances. See examples, characteristics, and a table of standard enthalpy of formation values for common substances. Learn how to use this table for enthalpy calculations and the points to remember for the process. In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in. The table covers alkanes, miscellaneous. Learn how to calculate enthalpy changes using standard enthalpies of formation, which are tabulated for many compounds under standard conditions. Find the molar heat of formation (δh f) of various compounds at 25 degrees celsius and one atom from elements in their stable form. Learn the definition, equation and examples of standard enthalpy of formation, the energy change when one mole of a substance is formed from. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. See examples, exercises, and a table of standard enthalpies of formation for common substances. Learn how to calculate or predict enthalpy changes for chemical reactions using standard enthalpy of formation, which is the enthalpy change when 1 mole of a pure substance forms from its elements. Find the standard enthalpy of formation, gibbs free energy of formation, entropy and heat capacity of 370 inorganic compounds at 25°c. Find heat of formation values for various compounds and elements in kcal/mol or kj/mol.
From
Standard Enthalpy Of Formation Table Find heat of formation values for various compounds and elements in kcal/mol or kj/mol. Learn how to calculate or predict enthalpy changes for chemical reactions using standard enthalpy of formation, which is the enthalpy change when 1 mole of a pure substance forms from its elements. Find the standard enthalpy of formation, gibbs free energy of formation, entropy and heat. Standard Enthalpy Of Formation Table.
From
Standard Enthalpy Of Formation Table In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in. The table covers alkanes, miscellaneous. See examples, characteristics, and a table of standard enthalpy of formation values for common substances. Learn how to calculate. Standard Enthalpy Of Formation Table.
From www.chegg.com
TABLE A286 Enthalpy of formation, Gibbs function of Standard Enthalpy Of Formation Table Learn how to calculate or predict enthalpy changes for chemical reactions using standard enthalpy of formation, which is the enthalpy change when 1 mole of a pure substance forms from its elements. Find heat of formation values for various compounds and elements in kcal/mol or kj/mol. See examples, characteristics, and a table of standard enthalpy of formation values for common. Standard Enthalpy Of Formation Table.
From
Standard Enthalpy Of Formation Table Find the standard enthalpy of formation, gibbs free energy of formation, entropy and heat capacity of 370 inorganic compounds at 25°c. Learn how to calculate or predict enthalpy changes for chemical reactions using standard enthalpy of formation, which is the enthalpy change when 1 mole of a pure substance forms from its elements. In chemistry and thermodynamics, the standard enthalpy. Standard Enthalpy Of Formation Table.
From
Standard Enthalpy Of Formation Table Learn the definition, equation and examples of standard enthalpy of formation, the energy change when one mole of a substance is formed from. See examples, characteristics, and a table of standard enthalpy of formation values for common substances. Learn how to calculate or predict enthalpy changes for chemical reactions using standard enthalpy of formation, which is the enthalpy change when. Standard Enthalpy Of Formation Table.
From
Standard Enthalpy Of Formation Table This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Find the molar heat of formation (δh f) of various compounds at 25 degrees celsius and one atom from elements in their stable form. Find heat of formation values for various compounds and elements in kcal/mol or. Standard Enthalpy Of Formation Table.
From
Standard Enthalpy Of Formation Table Find the standard enthalpy of formation, gibbs free energy of formation, entropy and heat capacity of 370 inorganic compounds at 25°c. See examples, exercises, and a table of standard enthalpies of formation for common substances. See examples, characteristics, and a table of standard enthalpy of formation values for common substances. Find heat of formation values for various compounds and elements. Standard Enthalpy Of Formation Table.
From
Standard Enthalpy Of Formation Table This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Learn how to calculate or predict enthalpy changes for chemical reactions using standard enthalpy of formation, which is the enthalpy change when 1 mole of a pure substance forms from its elements. Find the standard enthalpy of. Standard Enthalpy Of Formation Table.
From www.researchgate.net
Heat of formation and enthalpy data for slag compounds Enthalpy of Standard Enthalpy Of Formation Table In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in. Learn how to calculate or predict enthalpy changes for chemical reactions using standard enthalpy of formation, which is the enthalpy change when 1 mole. Standard Enthalpy Of Formation Table.
From
Standard Enthalpy Of Formation Table Learn how to use this table for enthalpy calculations and the points to remember for the process. Find the standard enthalpy of formation, gibbs free energy of formation, entropy and heat capacity of 370 inorganic compounds at 25°c. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and. Standard Enthalpy Of Formation Table.
From mungfali.com
Enthalpies Of Formation Table Standard Enthalpy Of Formation Table See examples, exercises, and a table of standard enthalpies of formation for common substances. Find the molar heat of formation (δh f) of various compounds at 25 degrees celsius and one atom from elements in their stable form. The table covers alkanes, miscellaneous. Find the standard enthalpy of formation, gibbs free energy of formation, entropy and heat capacity of 370. Standard Enthalpy Of Formation Table.
From
Standard Enthalpy Of Formation Table Learn how to calculate enthalpy changes using standard enthalpies of formation, which are tabulated for many compounds under standard conditions. See examples, exercises, and a table of standard enthalpies of formation for common substances. Learn how to use this table for enthalpy calculations and the points to remember for the process. Find heat of formation values for various compounds and. Standard Enthalpy Of Formation Table.
From
Standard Enthalpy Of Formation Table Learn how to calculate or predict enthalpy changes for chemical reactions using standard enthalpy of formation, which is the enthalpy change when 1 mole of a pure substance forms from its elements. Find the molar heat of formation (δh f) of various compounds at 25 degrees celsius and one atom from elements in their stable form. See examples, characteristics, and. Standard Enthalpy Of Formation Table.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Table Learn how to calculate or predict enthalpy changes for chemical reactions using standard enthalpy of formation, which is the enthalpy change when 1 mole of a pure substance forms from its elements. See examples, exercises, and a table of standard enthalpies of formation for common substances. Find heat of formation values for various compounds and elements in kcal/mol or kj/mol.. Standard Enthalpy Of Formation Table.
From people.chem.umass.edu
to Adobe GoLive 6 Standard Enthalpy Of Formation Table Find the standard enthalpy of formation, gibbs free energy of formation, entropy and heat capacity of 370 inorganic compounds at 25°c. Find heat of formation values for various compounds and elements in kcal/mol or kj/mol. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. See examples,. Standard Enthalpy Of Formation Table.
From slidesharenow.blogspot.com
Standard Gibbs Free Energy Of Formation Table slideshare Standard Enthalpy Of Formation Table This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Find heat of formation values for various compounds and elements in kcal/mol or kj/mol. See examples, exercises, and a table of standard enthalpies of formation for common substances. See examples, characteristics, and a table of standard enthalpy. Standard Enthalpy Of Formation Table.
From www.chegg.com
Look up the enthalpies of fusion and the melting Standard Enthalpy Of Formation Table See examples, characteristics, and a table of standard enthalpy of formation values for common substances. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Find heat of formation values for various compounds and elements in kcal/mol or kj/mol. Find the molar heat of formation (δh f). Standard Enthalpy Of Formation Table.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation Standard Enthalpy Of Formation Table Learn how to calculate enthalpy changes using standard enthalpies of formation, which are tabulated for many compounds under standard conditions. Find the molar heat of formation (δh f) of various compounds at 25 degrees celsius and one atom from elements in their stable form. Find heat of formation values for various compounds and elements in kcal/mol or kj/mol. Find the. Standard Enthalpy Of Formation Table.
From
Standard Enthalpy Of Formation Table See examples, characteristics, and a table of standard enthalpy of formation values for common substances. Find the standard enthalpy of formation, gibbs free energy of formation, entropy and heat capacity of 370 inorganic compounds at 25°c. Learn how to calculate or predict enthalpy changes for chemical reactions using standard enthalpy of formation, which is the enthalpy change when 1 mole. Standard Enthalpy Of Formation Table.
From
Standard Enthalpy Of Formation Table Learn how to calculate enthalpy changes using standard enthalpies of formation, which are tabulated for many compounds under standard conditions. Learn how to use this table for enthalpy calculations and the points to remember for the process. Learn the definition, equation and examples of standard enthalpy of formation, the energy change when one mole of a substance is formed from.. Standard Enthalpy Of Formation Table.
From
Standard Enthalpy Of Formation Table Learn the definition, equation and examples of standard enthalpy of formation, the energy change when one mole of a substance is formed from. Learn how to use this table for enthalpy calculations and the points to remember for the process. Find heat of formation values for various compounds and elements in kcal/mol or kj/mol. In chemistry and thermodynamics, the standard. Standard Enthalpy Of Formation Table.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Table Learn the definition, equation and examples of standard enthalpy of formation, the energy change when one mole of a substance is formed from. In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in. The. Standard Enthalpy Of Formation Table.
From
Standard Enthalpy Of Formation Table Learn how to calculate enthalpy changes using standard enthalpies of formation, which are tabulated for many compounds under standard conditions. Find the standard enthalpy of formation, gibbs free energy of formation, entropy and heat capacity of 370 inorganic compounds at 25°c. The table covers alkanes, miscellaneous. See examples, characteristics, and a table of standard enthalpy of formation values for common. Standard Enthalpy Of Formation Table.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Table This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Find the molar heat of formation (δh f) of various compounds at 25 degrees celsius and one atom from elements in their stable form. Learn how to calculate enthalpy changes using standard enthalpies of formation, which are. Standard Enthalpy Of Formation Table.
From
Standard Enthalpy Of Formation Table Learn how to calculate or predict enthalpy changes for chemical reactions using standard enthalpy of formation, which is the enthalpy change when 1 mole of a pure substance forms from its elements. See examples, exercises, and a table of standard enthalpies of formation for common substances. In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation. Standard Enthalpy Of Formation Table.
From
Standard Enthalpy Of Formation Table See examples, exercises, and a table of standard enthalpies of formation for common substances. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Learn the definition, equation and examples of standard enthalpy of formation, the energy change when one mole of a substance is formed from.. Standard Enthalpy Of Formation Table.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work Standard Enthalpy Of Formation Table The table covers alkanes, miscellaneous. Find the standard enthalpy of formation, gibbs free energy of formation, entropy and heat capacity of 370 inorganic compounds at 25°c. In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent. Standard Enthalpy Of Formation Table.
From
Standard Enthalpy Of Formation Table Find heat of formation values for various compounds and elements in kcal/mol or kj/mol. The table covers alkanes, miscellaneous. Learn how to calculate enthalpy changes using standard enthalpies of formation, which are tabulated for many compounds under standard conditions. In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard Enthalpy Of Formation Table.
From
Standard Enthalpy Of Formation Table See examples, exercises, and a table of standard enthalpies of formation for common substances. Find the molar heat of formation (δh f) of various compounds at 25 degrees celsius and one atom from elements in their stable form. In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy. Standard Enthalpy Of Formation Table.
From www.semanticscholar.org
Table II from Largescale calculations of gas phase thermochemistry Standard Enthalpy Of Formation Table Learn the definition, equation and examples of standard enthalpy of formation, the energy change when one mole of a substance is formed from. Find heat of formation values for various compounds and elements in kcal/mol or kj/mol. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the.. Standard Enthalpy Of Formation Table.
From darkataxa.blogspot.com
Astounding Collections Of Heat Of Formation Table Photos Darkata Standard Enthalpy Of Formation Table In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in. The table covers alkanes, miscellaneous. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. Standard Enthalpy Of Formation Table.
From stahonorschemistry.weebly.com
III Calculating Enthalpies STA Form IV Honors Chemistry Standard Enthalpy Of Formation Table See examples, characteristics, and a table of standard enthalpy of formation values for common substances. Find heat of formation values for various compounds and elements in kcal/mol or kj/mol. Find the molar heat of formation (δh f) of various compounds at 25 degrees celsius and one atom from elements in their stable form. Learn how to use this table for. Standard Enthalpy Of Formation Table.
From www.chegg.com
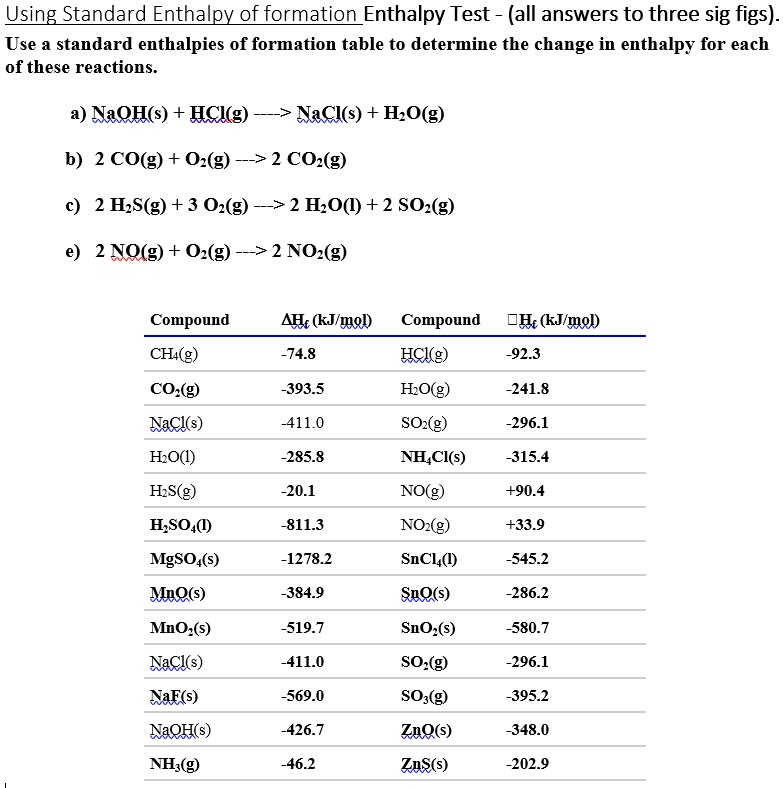
Solved Use a standard enthalpies of formation table to Standard Enthalpy Of Formation Table Learn how to calculate or predict enthalpy changes for chemical reactions using standard enthalpy of formation, which is the enthalpy change when 1 mole of a pure substance forms from its elements. Find the molar heat of formation (δh f) of various compounds at 25 degrees celsius and one atom from elements in their stable form. In chemistry and thermodynamics,. Standard Enthalpy Of Formation Table.
From
Standard Enthalpy Of Formation Table Find heat of formation values for various compounds and elements in kcal/mol or kj/mol. Learn how to use this table for enthalpy calculations and the points to remember for the process. Find the molar heat of formation (δh f) of various compounds at 25 degrees celsius and one atom from elements in their stable form. This table lists the standard. Standard Enthalpy Of Formation Table.
From
Standard Enthalpy Of Formation Table The table covers alkanes, miscellaneous. In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in. Find heat of formation values for various compounds and elements in kcal/mol or kj/mol. Learn how to calculate enthalpy. Standard Enthalpy Of Formation Table.