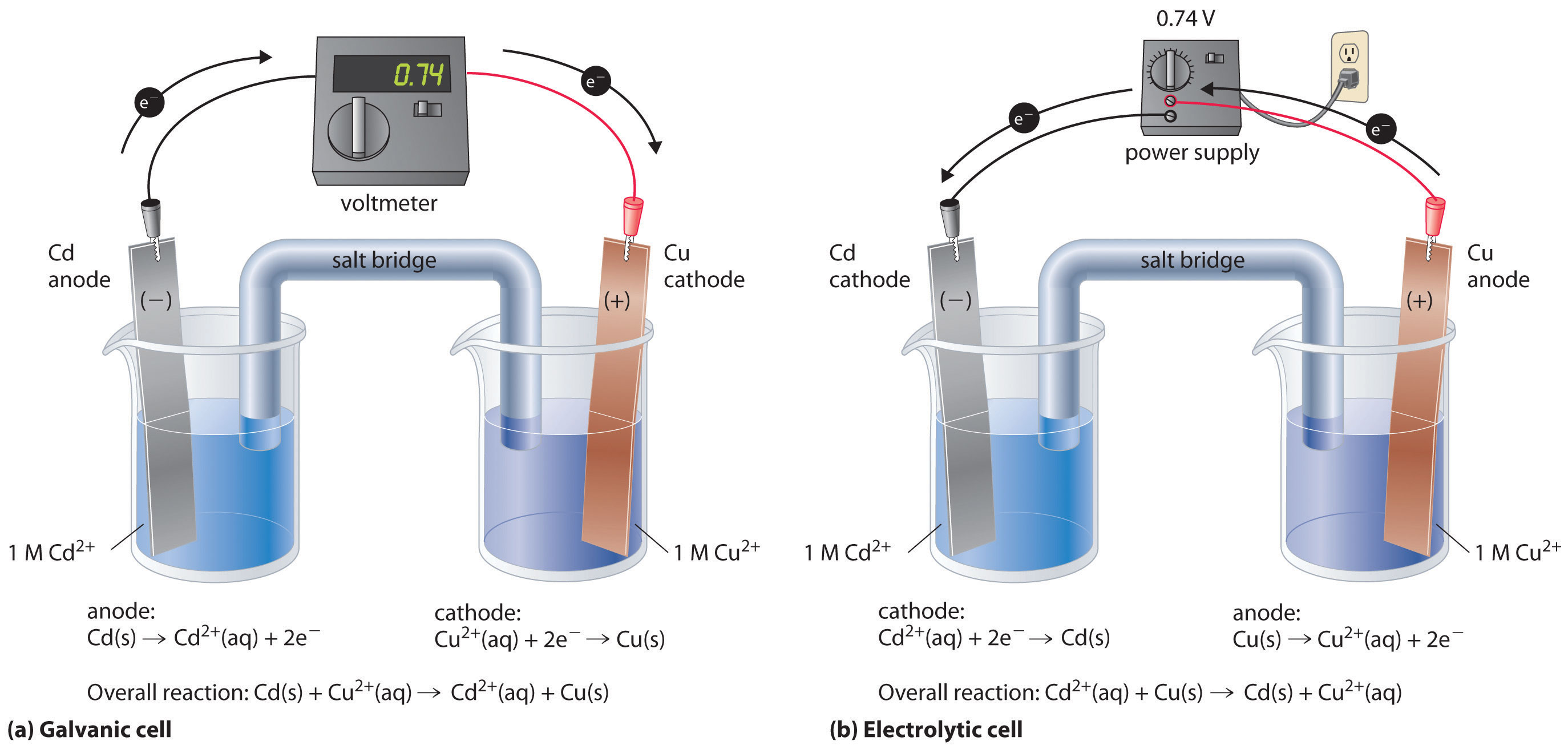
Electrodes For Electrolytic Cell . Learn about electrolysis, the decomposition of a substance by an electric current, and faraday's law, which relates the amount of substance produced to the charge passed. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Learn about electrolytic cells, which are the opposite of galvanic cells in terms of energy conversion and electron flow. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. The anode is defined as the electrode where oxidation occurs. Learn how to build and use electrochemical cells, which are devices that split redox reactions into half reactions and allow. Find examples, applications, and limitations of electrolysis in aqueous and molten solutions. Such a cell typically consists of. The cathode is the electrode where.
from chem.libretexts.org
Learn how to build and use electrochemical cells, which are devices that split redox reactions into half reactions and allow. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Such a cell typically consists of. Find examples, applications, and limitations of electrolysis in aqueous and molten solutions. Learn about electrolysis, the decomposition of a substance by an electric current, and faraday's law, which relates the amount of substance produced to the charge passed. The cathode is the electrode where. Learn about electrolytic cells, which are the opposite of galvanic cells in terms of energy conversion and electron flow. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. The anode is defined as the electrode where oxidation occurs.
Chapter 19.7 Electrolysis Chemistry LibreTexts
Electrodes For Electrolytic Cell Find examples, applications, and limitations of electrolysis in aqueous and molten solutions. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Find examples, applications, and limitations of electrolysis in aqueous and molten solutions. Learn about electrolysis, the decomposition of a substance by an electric current, and faraday's law, which relates the amount of substance produced to the charge passed. Learn how to build and use electrochemical cells, which are devices that split redox reactions into half reactions and allow. The anode is defined as the electrode where oxidation occurs. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. The cathode is the electrode where. Such a cell typically consists of. Learn about electrolytic cells, which are the opposite of galvanic cells in terms of energy conversion and electron flow.
From www.aooser.com
Lithium Ion Battery Equipment Threeelectrode Sealed electrodes Electrodes For Electrolytic Cell Find examples, applications, and limitations of electrolysis in aqueous and molten solutions. Such a cell typically consists of. The cathode is the electrode where. Learn about electrolytic cells, which are the opposite of galvanic cells in terms of energy conversion and electron flow. Electrochemical cells have two conductive electrodes, called the anode and the cathode. The anode is defined as. Electrodes For Electrolytic Cell.
From ar.inspiredpencil.com
Copper Electrolytic Cell Electrodes For Electrolytic Cell Find examples, applications, and limitations of electrolysis in aqueous and molten solutions. Learn about electrolytic cells, which are the opposite of galvanic cells in terms of energy conversion and electron flow. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Learn about electrolysis, the decomposition of a substance by an electric current, and faraday's law, which relates. Electrodes For Electrolytic Cell.
From hxekpjyly.blob.core.windows.net
Electrodes Current Definition at Tammi Alston blog Electrodes For Electrolytic Cell The cathode is the electrode where. The anode is defined as the electrode where oxidation occurs. Find examples, applications, and limitations of electrolysis in aqueous and molten solutions. Learn how to build and use electrochemical cells, which are devices that split redox reactions into half reactions and allow. Learn about electrolytic cells, which are the opposite of galvanic cells in. Electrodes For Electrolytic Cell.
From stock.adobe.com
Vector scientific illustration of the electrolysis processes. Set of Electrodes For Electrolytic Cell Learn how to build and use electrochemical cells, which are devices that split redox reactions into half reactions and allow. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. The anode is defined as the electrode where oxidation occurs. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Learn about. Electrodes For Electrolytic Cell.
From socratic.org
The following cell is operated as an electrolytic cell, using a current Electrodes For Electrolytic Cell The cathode is the electrode where. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Learn about electrolytic cells, which are the opposite of galvanic cells in terms of energy conversion and electron flow. Learn about electrolysis, the decomposition of a. Electrodes For Electrolytic Cell.
From app.pandai.org
Electrolytic Cell Electrodes For Electrolytic Cell Learn how to build and use electrochemical cells, which are devices that split redox reactions into half reactions and allow. Learn about electrolytic cells, which are the opposite of galvanic cells in terms of energy conversion and electron flow. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. The cathode is the electrode. Electrodes For Electrolytic Cell.
From www.expii.com
Electrochemical Cell — Definition & Overview Expii Electrodes For Electrolytic Cell Such a cell typically consists of. Learn about electrolytic cells, which are the opposite of galvanic cells in terms of energy conversion and electron flow. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. The cathode is the electrode where. Find examples, applications, and limitations of electrolysis in aqueous and molten solutions. Learn. Electrodes For Electrolytic Cell.
From www.differencebetween.com
What is the Difference Between Voltaic Cell and Electrolytic Cell Electrodes For Electrolytic Cell Learn about electrolytic cells, which are the opposite of galvanic cells in terms of energy conversion and electron flow. Such a cell typically consists of. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. Learn about electrolysis, the decomposition of a substance by an electric current, and faraday's law, which relates the amount. Electrodes For Electrolytic Cell.
From brainly.com
Please answer this! What are the halfreactions for electrolytic cell Electrodes For Electrolytic Cell Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. Such a cell typically consists of. Learn about electrolysis, the decomposition of a substance by an electric current, and faraday's law, which relates the amount of substance produced to the charge passed. Learn how to build and use electrochemical cells, which are devices that. Electrodes For Electrolytic Cell.
From www.alamy.com
Electrolysis hires stock photography and images Alamy Electrodes For Electrolytic Cell Electrochemical cells have two conductive electrodes, called the anode and the cathode. The cathode is the electrode where. Learn how to build and use electrochemical cells, which are devices that split redox reactions into half reactions and allow. The anode is defined as the electrode where oxidation occurs. Learn about electrolysis, the decomposition of a substance by an electric current,. Electrodes For Electrolytic Cell.
From courses.lumenlearning.com
Predicting the Products of Electrolysis Introduction to Chemistry Electrodes For Electrolytic Cell Electrochemical cells have two conductive electrodes, called the anode and the cathode. Learn about electrolysis, the decomposition of a substance by an electric current, and faraday's law, which relates the amount of substance produced to the charge passed. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. The cathode is the electrode where.. Electrodes For Electrolytic Cell.
From www.bajeczneobrazy.pl
Electrolytic cell infographic diagram with components including anode Electrodes For Electrolytic Cell The cathode is the electrode where. Learn how to build and use electrochemical cells, which are devices that split redox reactions into half reactions and allow. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. Such a cell typically consists of. Learn about electrolytic cells, which are the opposite of galvanic cells in. Electrodes For Electrolytic Cell.
From www.aotelec.com
Buy Threeelectrode Sealed Electrodes Electrolytic Cell For Laboratory Electrodes For Electrolytic Cell The anode is defined as the electrode where oxidation occurs. Learn about electrolysis, the decomposition of a substance by an electric current, and faraday's law, which relates the amount of substance produced to the charge passed. Learn about electrolytic cells, which are the opposite of galvanic cells in terms of energy conversion and electron flow. Find examples, applications, and limitations. Electrodes For Electrolytic Cell.
From byjus.com
An electrolytic cell is used to convert Electrodes For Electrolytic Cell The cathode is the electrode where. Such a cell typically consists of. Find examples, applications, and limitations of electrolysis in aqueous and molten solutions. The anode is defined as the electrode where oxidation occurs. Learn about electrolysis, the decomposition of a substance by an electric current, and faraday's law, which relates the amount of substance produced to the charge passed.. Electrodes For Electrolytic Cell.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Electrodes For Electrolytic Cell The cathode is the electrode where. Such a cell typically consists of. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Learn about electrolysis, the decomposition of a substance by an electric current, and faraday's law, which relates the amount of substance produced to the charge passed. Learn about electrolytic cells, which are the opposite of galvanic. Electrodes For Electrolytic Cell.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Electrodes For Electrolytic Cell Such a cell typically consists of. The cathode is the electrode where. Learn about electrolysis, the decomposition of a substance by an electric current, and faraday's law, which relates the amount of substance produced to the charge passed. The anode is defined as the electrode where oxidation occurs. Electrochemical cells have two conductive electrodes, called the anode and the cathode.. Electrodes For Electrolytic Cell.
From www.researchgate.net
Electrolytic cell and electrodes a counter electrode; b reference Electrodes For Electrolytic Cell Learn how to build and use electrochemical cells, which are devices that split redox reactions into half reactions and allow. Find examples, applications, and limitations of electrolysis in aqueous and molten solutions. Learn about electrolysis, the decomposition of a substance by an electric current, and faraday's law, which relates the amount of substance produced to the charge passed. Learn about. Electrodes For Electrolytic Cell.
From ar.inspiredpencil.com
Copper Electrolytic Cell Electrodes For Electrolytic Cell Learn about electrolytic cells, which are the opposite of galvanic cells in terms of energy conversion and electron flow. Such a cell typically consists of. The cathode is the electrode where. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. Learn how to build and use electrochemical cells, which are devices that split. Electrodes For Electrolytic Cell.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrodes For Electrolytic Cell Electrochemical cells have two conductive electrodes, called the anode and the cathode. The cathode is the electrode where. Such a cell typically consists of. Learn about electrolysis, the decomposition of a substance by an electric current, and faraday's law, which relates the amount of substance produced to the charge passed. Learn about electrolytic cells, which are the opposite of galvanic. Electrodes For Electrolytic Cell.
From ar.inspiredpencil.com
Copper Electrolytic Cell Electrodes For Electrolytic Cell Find examples, applications, and limitations of electrolysis in aqueous and molten solutions. The anode is defined as the electrode where oxidation occurs. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. Learn about electrolytic cells, which are the opposite of galvanic cells in terms of energy conversion and electron flow. Electrochemical cells have. Electrodes For Electrolytic Cell.
From www.researchgate.net
Front view of the electrolytic cell used for the experiments. The cell Electrodes For Electrolytic Cell Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. Learn about electrolysis, the decomposition of a substance by an electric current, and faraday's law, which relates the amount of substance produced to the charge passed. Learn about electrolytic cells, which are the opposite of galvanic cells in terms of energy conversion and electron. Electrodes For Electrolytic Cell.
From chem.libretexts.org
5.6 Day 41 Electrolysis; Commercial Batteries Chemistry LibreTexts Electrodes For Electrolytic Cell Find examples, applications, and limitations of electrolysis in aqueous and molten solutions. Electrochemical cells have two conductive electrodes, called the anode and the cathode. The anode is defined as the electrode where oxidation occurs. The cathode is the electrode where. Such a cell typically consists of. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or. Electrodes For Electrolytic Cell.
From favpng.com
Electrolytic Cell Electrolysis Of Water Electricity Electrochemistry Electrodes For Electrolytic Cell The anode is defined as the electrode where oxidation occurs. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Such a cell typically consists of. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. The cathode is the electrode where. Learn about electrolysis, the decomposition of a substance by an. Electrodes For Electrolytic Cell.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo Electrodes For Electrolytic Cell Learn how to build and use electrochemical cells, which are devices that split redox reactions into half reactions and allow. Such a cell typically consists of. Learn about electrolysis, the decomposition of a substance by an electric current, and faraday's law, which relates the amount of substance produced to the charge passed. Learn about electrolytic cells, which are the opposite. Electrodes For Electrolytic Cell.
From socratic.org
What is a voltaic cell? + Example Electrodes For Electrolytic Cell Learn how to build and use electrochemical cells, which are devices that split redox reactions into half reactions and allow. Find examples, applications, and limitations of electrolysis in aqueous and molten solutions. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. The cathode is the electrode where. Electrochemical cells have two conductive electrodes,. Electrodes For Electrolytic Cell.
From www.mdpi.com
Catalysts Free FullText Fundamentals of Gas Diffusion Electrodes Electrodes For Electrolytic Cell Electrochemical cells have two conductive electrodes, called the anode and the cathode. Find examples, applications, and limitations of electrolysis in aqueous and molten solutions. The anode is defined as the electrode where oxidation occurs. Learn how to build and use electrochemical cells, which are devices that split redox reactions into half reactions and allow. Learn about electrolytic cells, which are. Electrodes For Electrolytic Cell.
From www.mdpi.com
Sensors Free FullText Microfabricated Reference Electrodes and Electrodes For Electrolytic Cell Such a cell typically consists of. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. Learn how to build and use electrochemical cells, which are devices that split redox reactions into half reactions and allow. Learn about electrolysis, the decomposition of. Electrodes For Electrolytic Cell.
From www.alamy.com
Electrolytic process Stock Vector Images Alamy Electrodes For Electrolytic Cell Learn how to build and use electrochemical cells, which are devices that split redox reactions into half reactions and allow. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. The anode is defined as the electrode where oxidation occurs. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Learn about. Electrodes For Electrolytic Cell.
From www.nagwa.com
Question Video Recalling the Name of the Positive Electrode in an Electrodes For Electrolytic Cell Learn how to build and use electrochemical cells, which are devices that split redox reactions into half reactions and allow. Such a cell typically consists of. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Learn about electrolysis, the decomposition of a substance by an electric current, and faraday's law, which relates the amount of substance produced. Electrodes For Electrolytic Cell.
From classnotes.org.in
Electrolytic Cells Chemistry, Class 12, Electro Chemistry Electrodes For Electrolytic Cell Learn about electrolytic cells, which are the opposite of galvanic cells in terms of energy conversion and electron flow. The anode is defined as the electrode where oxidation occurs. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Learn about electrolysis,. Electrodes For Electrolytic Cell.
From www.aotbattery.com
Electrolytic Cell for Electrochemistry Electrodes For Electrolytic Cell Electrochemical cells have two conductive electrodes, called the anode and the cathode. The anode is defined as the electrode where oxidation occurs. Learn how to build and use electrochemical cells, which are devices that split redox reactions into half reactions and allow. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. The cathode. Electrodes For Electrolytic Cell.
From quizizz.com
Electrolytic Cell 385 plays Quizizz Electrodes For Electrolytic Cell Find examples, applications, and limitations of electrolysis in aqueous and molten solutions. Learn about electrolytic cells, which are the opposite of galvanic cells in terms of energy conversion and electron flow. Learn about electrolysis, the decomposition of a substance by an electric current, and faraday's law, which relates the amount of substance produced to the charge passed. Electrolytic cell, any. Electrodes For Electrolytic Cell.
From www.pinterest.com
Electrolytic cell Electrolysis of NaCl Cell definition, Chemistry Electrodes For Electrolytic Cell Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. Find examples, applications, and limitations of electrolysis in aqueous and molten solutions. Learn about electrolytic cells, which are the opposite of galvanic cells in terms of energy conversion and electron flow. Electrochemical cells have two conductive electrodes, called the anode and the cathode. The. Electrodes For Electrolytic Cell.
From flowerwallpapersforiphonexr.blogspot.com
draw the diagram of electrolytic cell and explain Electrodes For Electrolytic Cell The cathode is the electrode where. Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. The anode is defined as the electrode where oxidation occurs. Learn how to build and use electrochemical cells, which are devices that split redox reactions into half reactions and allow. Learn about electrolytic cells, which are the opposite. Electrodes For Electrolytic Cell.
From www.researchgate.net
A divided laboratory electrolysis cell using planar electrodes using Electrodes For Electrolytic Cell Find examples, applications, and limitations of electrolysis in aqueous and molten solutions. Learn about electrolysis, the decomposition of a substance by an electric current, and faraday's law, which relates the amount of substance produced to the charge passed. Learn about electrolytic cells, which are the opposite of galvanic cells in terms of energy conversion and electron flow. The cathode is. Electrodes For Electrolytic Cell.