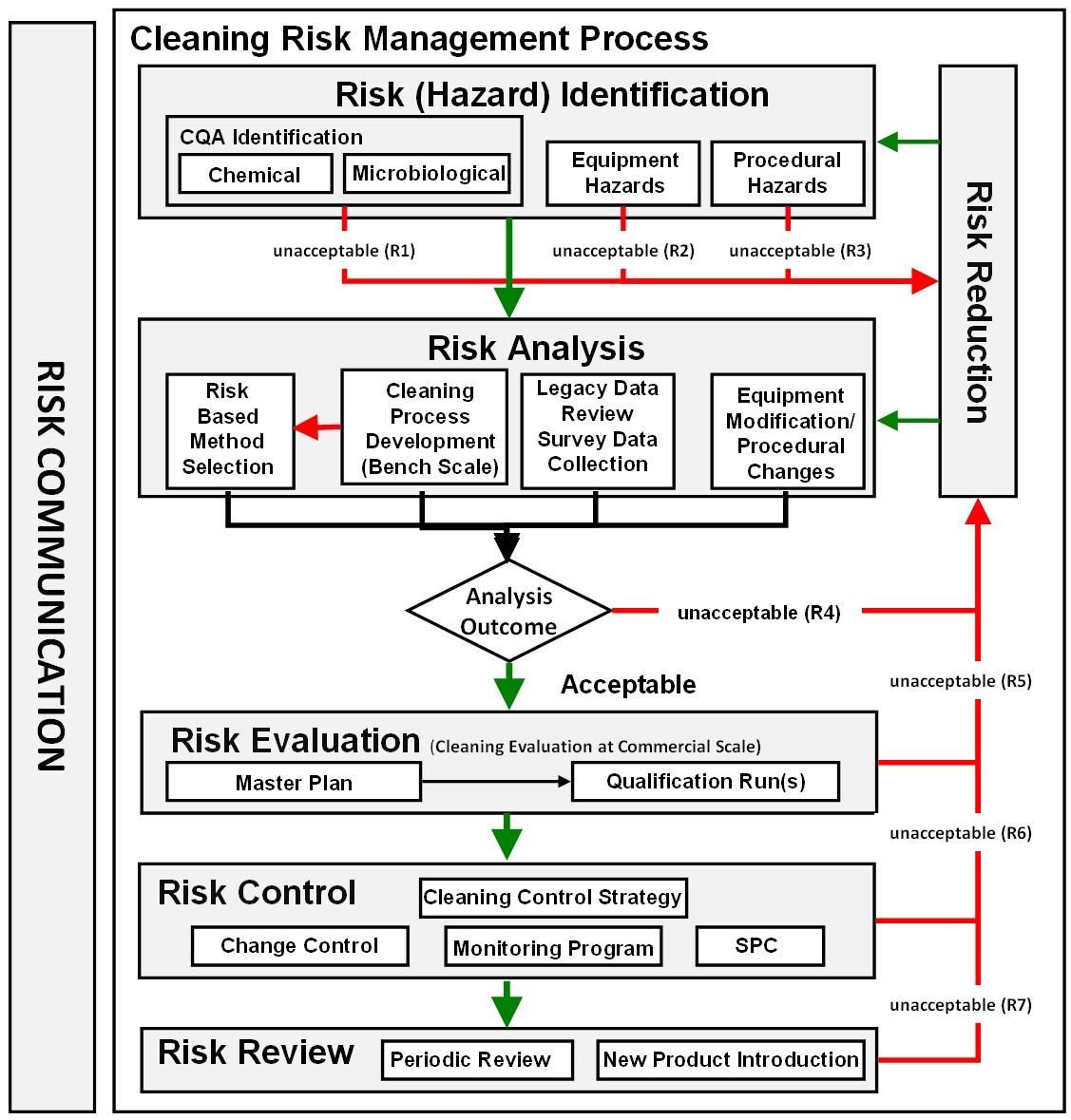
Safety Factor In Cleaning Validation . Cleaning validation requirements could be fulfilled and implemented as part of routine operations. Cleaning validation and gmp requirements. Sf safety factor the safety factor (sf) varies depending on the route of administration. Emmett broderick gmp inspector, manufacturing quality branch, tga. This safety factor ensures that the. In addition, apic has aligned this guidance with. Generally a factor of 200 is employed when. In the pda tr29 2012, it is mentionned that the security factor applied to the ld50 can’t no more than 1 000 000. It explains the concepts of safety factor, minimum batch size,. A presentation on the history and guidelines of cleaning validation and risk assessment for active pharmaceutical ingredients. To account for variability and uncertainties, a safety factor is applied to the noel.
from www.pharmaceuticalonline.com
Generally a factor of 200 is employed when. This safety factor ensures that the. Cleaning validation requirements could be fulfilled and implemented as part of routine operations. Cleaning validation and gmp requirements. A presentation on the history and guidelines of cleaning validation and risk assessment for active pharmaceutical ingredients. It explains the concepts of safety factor, minimum batch size,. To account for variability and uncertainties, a safety factor is applied to the noel. In the pda tr29 2012, it is mentionned that the security factor applied to the ld50 can’t no more than 1 000 000. Emmett broderick gmp inspector, manufacturing quality branch, tga. Sf safety factor the safety factor (sf) varies depending on the route of administration.
Introduction To Science And RiskBased Cleaning Validation Using ASTM
Safety Factor In Cleaning Validation Generally a factor of 200 is employed when. In addition, apic has aligned this guidance with. In the pda tr29 2012, it is mentionned that the security factor applied to the ld50 can’t no more than 1 000 000. Cleaning validation and gmp requirements. Generally a factor of 200 is employed when. Cleaning validation requirements could be fulfilled and implemented as part of routine operations. This safety factor ensures that the. A presentation on the history and guidelines of cleaning validation and risk assessment for active pharmaceutical ingredients. It explains the concepts of safety factor, minimum batch size,. Emmett broderick gmp inspector, manufacturing quality branch, tga. To account for variability and uncertainties, a safety factor is applied to the noel. Sf safety factor the safety factor (sf) varies depending on the route of administration.
From dokumen.tips
(PPT) Factor of Safety (Safety Factor) In the calculations, Material Safety Factor In Cleaning Validation Cleaning validation and gmp requirements. A presentation on the history and guidelines of cleaning validation and risk assessment for active pharmaceutical ingredients. To account for variability and uncertainties, a safety factor is applied to the noel. Generally a factor of 200 is employed when. Sf safety factor the safety factor (sf) varies depending on the route of administration. This safety. Safety Factor In Cleaning Validation.
From www.youtube.com
Cleaning Validation for API YouTube Safety Factor In Cleaning Validation Generally a factor of 200 is employed when. This safety factor ensures that the. A presentation on the history and guidelines of cleaning validation and risk assessment for active pharmaceutical ingredients. Cleaning validation requirements could be fulfilled and implemented as part of routine operations. In addition, apic has aligned this guidance with. To account for variability and uncertainties, a safety. Safety Factor In Cleaning Validation.
From www.pharmacalculation.com
MACO Calculation in Cleaning Validation Safety Factor In Cleaning Validation This safety factor ensures that the. Sf safety factor the safety factor (sf) varies depending on the route of administration. Generally a factor of 200 is employed when. Cleaning validation requirements could be fulfilled and implemented as part of routine operations. Cleaning validation and gmp requirements. A presentation on the history and guidelines of cleaning validation and risk assessment for. Safety Factor In Cleaning Validation.
From www.slideserve.com
PPT Electrical Safety in Construction PowerPoint Presentation, free Safety Factor In Cleaning Validation A presentation on the history and guidelines of cleaning validation and risk assessment for active pharmaceutical ingredients. Cleaning validation and gmp requirements. Emmett broderick gmp inspector, manufacturing quality branch, tga. This safety factor ensures that the. Cleaning validation requirements could be fulfilled and implemented as part of routine operations. In the pda tr29 2012, it is mentionned that the security. Safety Factor In Cleaning Validation.
From www.researchgate.net
(PDF) CLEANING VALIDATION IN PHARMACEUTICAL INDUSTRIES Safety Factor In Cleaning Validation In the pda tr29 2012, it is mentionned that the security factor applied to the ld50 can’t no more than 1 000 000. In addition, apic has aligned this guidance with. Generally a factor of 200 is employed when. This safety factor ensures that the. Cleaning validation requirements could be fulfilled and implemented as part of routine operations. It explains. Safety Factor In Cleaning Validation.
From pharmaceuticalupdates.com
Cleaning Validation in Pharmaceutical Industry Pharmaceutical Updates Safety Factor In Cleaning Validation Generally a factor of 200 is employed when. To account for variability and uncertainties, a safety factor is applied to the noel. Cleaning validation requirements could be fulfilled and implemented as part of routine operations. It explains the concepts of safety factor, minimum batch size,. This safety factor ensures that the. Emmett broderick gmp inspector, manufacturing quality branch, tga. In. Safety Factor In Cleaning Validation.
From slideplayer.com
CLEANING VALIDATION BASIC CONCEPTS. ppt download Safety Factor In Cleaning Validation This safety factor ensures that the. A presentation on the history and guidelines of cleaning validation and risk assessment for active pharmaceutical ingredients. In addition, apic has aligned this guidance with. It explains the concepts of safety factor, minimum batch size,. Sf safety factor the safety factor (sf) varies depending on the route of administration. Cleaning validation and gmp requirements.. Safety Factor In Cleaning Validation.
From www.presentationeze.com
Cleaning Validation PresentationEZE Safety Factor In Cleaning Validation Emmett broderick gmp inspector, manufacturing quality branch, tga. Cleaning validation and gmp requirements. Generally a factor of 200 is employed when. Cleaning validation requirements could be fulfilled and implemented as part of routine operations. Sf safety factor the safety factor (sf) varies depending on the route of administration. In addition, apic has aligned this guidance with. In the pda tr29. Safety Factor In Cleaning Validation.
From www.pharmaspecialists.com
Cleaning Validation in Pharmaceutical Industry Safety Factor In Cleaning Validation Cleaning validation and gmp requirements. This safety factor ensures that the. It explains the concepts of safety factor, minimum batch size,. In the pda tr29 2012, it is mentionned that the security factor applied to the ld50 can’t no more than 1 000 000. A presentation on the history and guidelines of cleaning validation and risk assessment for active pharmaceutical. Safety Factor In Cleaning Validation.
From www.slideserve.com
PPT Cleaning Validation PowerPoint Presentation, free download ID Safety Factor In Cleaning Validation A presentation on the history and guidelines of cleaning validation and risk assessment for active pharmaceutical ingredients. Sf safety factor the safety factor (sf) varies depending on the route of administration. In the pda tr29 2012, it is mentionned that the security factor applied to the ld50 can’t no more than 1 000 000. Cleaning validation and gmp requirements. Emmett. Safety Factor In Cleaning Validation.
From www.youtube.com
How to establish MACO Value during cleaning validation YouTube Safety Factor In Cleaning Validation Cleaning validation requirements could be fulfilled and implemented as part of routine operations. In the pda tr29 2012, it is mentionned that the security factor applied to the ld50 can’t no more than 1 000 000. In addition, apic has aligned this guidance with. Cleaning validation and gmp requirements. Emmett broderick gmp inspector, manufacturing quality branch, tga. This safety factor. Safety Factor In Cleaning Validation.
From www.scribd.com
Factor of Safety PDF Safety Factor In Cleaning Validation In the pda tr29 2012, it is mentionned that the security factor applied to the ld50 can’t no more than 1 000 000. Sf safety factor the safety factor (sf) varies depending on the route of administration. Cleaning validation and gmp requirements. Emmett broderick gmp inspector, manufacturing quality branch, tga. A presentation on the history and guidelines of cleaning validation. Safety Factor In Cleaning Validation.
From www.pdfprof.com
Présentation. Procédure Safety Factor In Cleaning Validation A presentation on the history and guidelines of cleaning validation and risk assessment for active pharmaceutical ingredients. Cleaning validation and gmp requirements. Sf safety factor the safety factor (sf) varies depending on the route of administration. Cleaning validation requirements could be fulfilled and implemented as part of routine operations. In the pda tr29 2012, it is mentionned that the security. Safety Factor In Cleaning Validation.
From pharmaceuticalsindex.com
Importance of Cleaning Validation Pharmaceuticals Index Safety Factor In Cleaning Validation Cleaning validation requirements could be fulfilled and implemented as part of routine operations. It explains the concepts of safety factor, minimum batch size,. Generally a factor of 200 is employed when. A presentation on the history and guidelines of cleaning validation and risk assessment for active pharmaceutical ingredients. In addition, apic has aligned this guidance with. Emmett broderick gmp inspector,. Safety Factor In Cleaning Validation.
From www.youtube.com
Cleaning Validation Part 1 MACO Calculation YouTube Safety Factor In Cleaning Validation Sf safety factor the safety factor (sf) varies depending on the route of administration. To account for variability and uncertainties, a safety factor is applied to the noel. In the pda tr29 2012, it is mentionned that the security factor applied to the ld50 can’t no more than 1 000 000. This safety factor ensures that the. Generally a factor. Safety Factor In Cleaning Validation.
From www.a3p.org
“Healthbased approach” implementation for setting limits in cleaning Safety Factor In Cleaning Validation This safety factor ensures that the. It explains the concepts of safety factor, minimum batch size,. Sf safety factor the safety factor (sf) varies depending on the route of administration. In addition, apic has aligned this guidance with. In the pda tr29 2012, it is mentionned that the security factor applied to the ld50 can’t no more than 1 000. Safety Factor In Cleaning Validation.
From www.youtube.com
cleaning validation YouTube Safety Factor In Cleaning Validation Generally a factor of 200 is employed when. Sf safety factor the safety factor (sf) varies depending on the route of administration. Cleaning validation requirements could be fulfilled and implemented as part of routine operations. A presentation on the history and guidelines of cleaning validation and risk assessment for active pharmaceutical ingredients. In addition, apic has aligned this guidance with.. Safety Factor In Cleaning Validation.
From www.youtube.com
NOEL and MACO Calculations Cleaning Validation Calculations YouTube Safety Factor In Cleaning Validation Sf safety factor the safety factor (sf) varies depending on the route of administration. Generally a factor of 200 is employed when. In addition, apic has aligned this guidance with. It explains the concepts of safety factor, minimum batch size,. Cleaning validation and gmp requirements. In the pda tr29 2012, it is mentionned that the security factor applied to the. Safety Factor In Cleaning Validation.
From gmpinsiders.com
Cleaning Validation In The Pharmaceutical Industry Safety Factor In Cleaning Validation In addition, apic has aligned this guidance with. A presentation on the history and guidelines of cleaning validation and risk assessment for active pharmaceutical ingredients. In the pda tr29 2012, it is mentionned that the security factor applied to the ld50 can’t no more than 1 000 000. Generally a factor of 200 is employed when. Cleaning validation requirements could. Safety Factor In Cleaning Validation.
From farmasiindustri.com
Perhitungan NOEL dan MACO pada validasi pembersihan FARMASI INDUSTRI Safety Factor In Cleaning Validation To account for variability and uncertainties, a safety factor is applied to the noel. In the pda tr29 2012, it is mentionned that the security factor applied to the ld50 can’t no more than 1 000 000. This safety factor ensures that the. In addition, apic has aligned this guidance with. Cleaning validation and gmp requirements. Generally a factor of. Safety Factor In Cleaning Validation.
From batubuayabradleys.blogspot.com
How To Calculate Factor Of Safety Safety Factor In Cleaning Validation Generally a factor of 200 is employed when. It explains the concepts of safety factor, minimum batch size,. Emmett broderick gmp inspector, manufacturing quality branch, tga. In the pda tr29 2012, it is mentionned that the security factor applied to the ld50 can’t no more than 1 000 000. A presentation on the history and guidelines of cleaning validation and. Safety Factor In Cleaning Validation.
From ntint.com
Cleaning Validation Frequently Asked Questions Novatek International Safety Factor In Cleaning Validation Generally a factor of 200 is employed when. Sf safety factor the safety factor (sf) varies depending on the route of administration. It explains the concepts of safety factor, minimum batch size,. In addition, apic has aligned this guidance with. A presentation on the history and guidelines of cleaning validation and risk assessment for active pharmaceutical ingredients. To account for. Safety Factor In Cleaning Validation.
From ciqa.net
All You Need to Know About Cleaning Validation • Download templates Safety Factor In Cleaning Validation A presentation on the history and guidelines of cleaning validation and risk assessment for active pharmaceutical ingredients. This safety factor ensures that the. Emmett broderick gmp inspector, manufacturing quality branch, tga. To account for variability and uncertainties, a safety factor is applied to the noel. Generally a factor of 200 is employed when. In the pda tr29 2012, it is. Safety Factor In Cleaning Validation.
From www.alten.pt
Product Carryover Limit and Active Substance (API) Worst Case based on Safety Factor In Cleaning Validation Cleaning validation requirements could be fulfilled and implemented as part of routine operations. Cleaning validation and gmp requirements. Generally a factor of 200 is employed when. In the pda tr29 2012, it is mentionned that the security factor applied to the ld50 can’t no more than 1 000 000. A presentation on the history and guidelines of cleaning validation and. Safety Factor In Cleaning Validation.
From www.gmp-journal.com
IMPACT OF THE CHANGES TO THE EUROPEAN GMPs ON CLEANING VALIDATION Safety Factor In Cleaning Validation A presentation on the history and guidelines of cleaning validation and risk assessment for active pharmaceutical ingredients. In the pda tr29 2012, it is mentionned that the security factor applied to the ld50 can’t no more than 1 000 000. Generally a factor of 200 is employed when. Cleaning validation requirements could be fulfilled and implemented as part of routine. Safety Factor In Cleaning Validation.
From www.slideserve.com
PPT Factor of Safety (Safety Factor) PowerPoint Presentation, free Safety Factor In Cleaning Validation A presentation on the history and guidelines of cleaning validation and risk assessment for active pharmaceutical ingredients. Cleaning validation and gmp requirements. Sf safety factor the safety factor (sf) varies depending on the route of administration. Emmett broderick gmp inspector, manufacturing quality branch, tga. Generally a factor of 200 is employed when. It explains the concepts of safety factor, minimum. Safety Factor In Cleaning Validation.
From safetyculture.com
Cleaning Validation Protocol & Guidelines SafetyCulture Safety Factor In Cleaning Validation Sf safety factor the safety factor (sf) varies depending on the route of administration. This safety factor ensures that the. Emmett broderick gmp inspector, manufacturing quality branch, tga. Cleaning validation requirements could be fulfilled and implemented as part of routine operations. To account for variability and uncertainties, a safety factor is applied to the noel. In the pda tr29 2012,. Safety Factor In Cleaning Validation.
From www.vrogue.co
Cleaning Validation In Pharmaceutical Industry Verifi vrogue.co Safety Factor In Cleaning Validation In the pda tr29 2012, it is mentionned that the security factor applied to the ld50 can’t no more than 1 000 000. A presentation on the history and guidelines of cleaning validation and risk assessment for active pharmaceutical ingredients. This safety factor ensures that the. Sf safety factor the safety factor (sf) varies depending on the route of administration.. Safety Factor In Cleaning Validation.
From www.youtube.com
Requirement and Reasons of Cleaning Validation! Basics of cleaning Safety Factor In Cleaning Validation Sf safety factor the safety factor (sf) varies depending on the route of administration. Cleaning validation requirements could be fulfilled and implemented as part of routine operations. In the pda tr29 2012, it is mentionned that the security factor applied to the ld50 can’t no more than 1 000 000. This safety factor ensures that the. To account for variability. Safety Factor In Cleaning Validation.
From www.pharmaceuticalonline.com
Introduction To Science And RiskBased Cleaning Validation Using ASTM Safety Factor In Cleaning Validation This safety factor ensures that the. Generally a factor of 200 is employed when. Sf safety factor the safety factor (sf) varies depending on the route of administration. It explains the concepts of safety factor, minimum batch size,. Cleaning validation and gmp requirements. In addition, apic has aligned this guidance with. Emmett broderick gmp inspector, manufacturing quality branch, tga. To. Safety Factor In Cleaning Validation.
From ntint.com
Cleaning Validation Frequently Asked Questions Novatek International Safety Factor In Cleaning Validation In addition, apic has aligned this guidance with. Cleaning validation and gmp requirements. In the pda tr29 2012, it is mentionned that the security factor applied to the ld50 can’t no more than 1 000 000. This safety factor ensures that the. Sf safety factor the safety factor (sf) varies depending on the route of administration. Cleaning validation requirements could. Safety Factor In Cleaning Validation.
From gmpinsiders.com
Worst Case Selection In Cleaning Validation Safety Factor In Cleaning Validation In addition, apic has aligned this guidance with. A presentation on the history and guidelines of cleaning validation and risk assessment for active pharmaceutical ingredients. Sf safety factor the safety factor (sf) varies depending on the route of administration. This safety factor ensures that the. In the pda tr29 2012, it is mentionned that the security factor applied to the. Safety Factor In Cleaning Validation.
From www.slideshare.net
Cleaning validation a complete know how Safety Factor In Cleaning Validation Emmett broderick gmp inspector, manufacturing quality branch, tga. This safety factor ensures that the. In the pda tr29 2012, it is mentionned that the security factor applied to the ld50 can’t no more than 1 000 000. Cleaning validation and gmp requirements. Cleaning validation requirements could be fulfilled and implemented as part of routine operations. To account for variability and. Safety Factor In Cleaning Validation.
From www.a3p.org
Toxicological approach to define the PDE for your cleaning validation Safety Factor In Cleaning Validation A presentation on the history and guidelines of cleaning validation and risk assessment for active pharmaceutical ingredients. Sf safety factor the safety factor (sf) varies depending on the route of administration. To account for variability and uncertainties, a safety factor is applied to the noel. It explains the concepts of safety factor, minimum batch size,. Cleaning validation requirements could be. Safety Factor In Cleaning Validation.
From www.slideshare.net
Basic Concepts of Cleaning validation Safety Factor In Cleaning Validation To account for variability and uncertainties, a safety factor is applied to the noel. In the pda tr29 2012, it is mentionned that the security factor applied to the ld50 can’t no more than 1 000 000. Emmett broderick gmp inspector, manufacturing quality branch, tga. Cleaning validation and gmp requirements. In addition, apic has aligned this guidance with. This safety. Safety Factor In Cleaning Validation.