Testing For Rate Of Reaction . To measure the rate of a reaction, we need to be able to measure either how quickly the reactants are used up or how quickly the products are formed. Learn how to measure reaction rates by monitoring the depletion of reactants or the formation of products. Find out how to calculate. Measuring the volume of gas given off by a reaction over time. There are three main methods of measuring rate: Firstly, it’s important to understand what a rate of reaction is. How do we monitor rates of reaction? Explore the contrast between thermodynamics and kinetics,. Describe the rate of a reaction in terms of change of a measureable quantity, with time; Learn how to measure the rate of reaction by colour change using sodium thiosulfate and hydrochloric acid. Identify and apply factors affecting rates of.
from www.linstitute.net
To measure the rate of a reaction, we need to be able to measure either how quickly the reactants are used up or how quickly the products are formed. Find out how to calculate. Learn how to measure reaction rates by monitoring the depletion of reactants or the formation of products. Measuring the volume of gas given off by a reaction over time. Learn how to measure the rate of reaction by colour change using sodium thiosulfate and hydrochloric acid. Firstly, it’s important to understand what a rate of reaction is. There are three main methods of measuring rate: Describe the rate of a reaction in terms of change of a measureable quantity, with time; How do we monitor rates of reaction? Explore the contrast between thermodynamics and kinetics,.
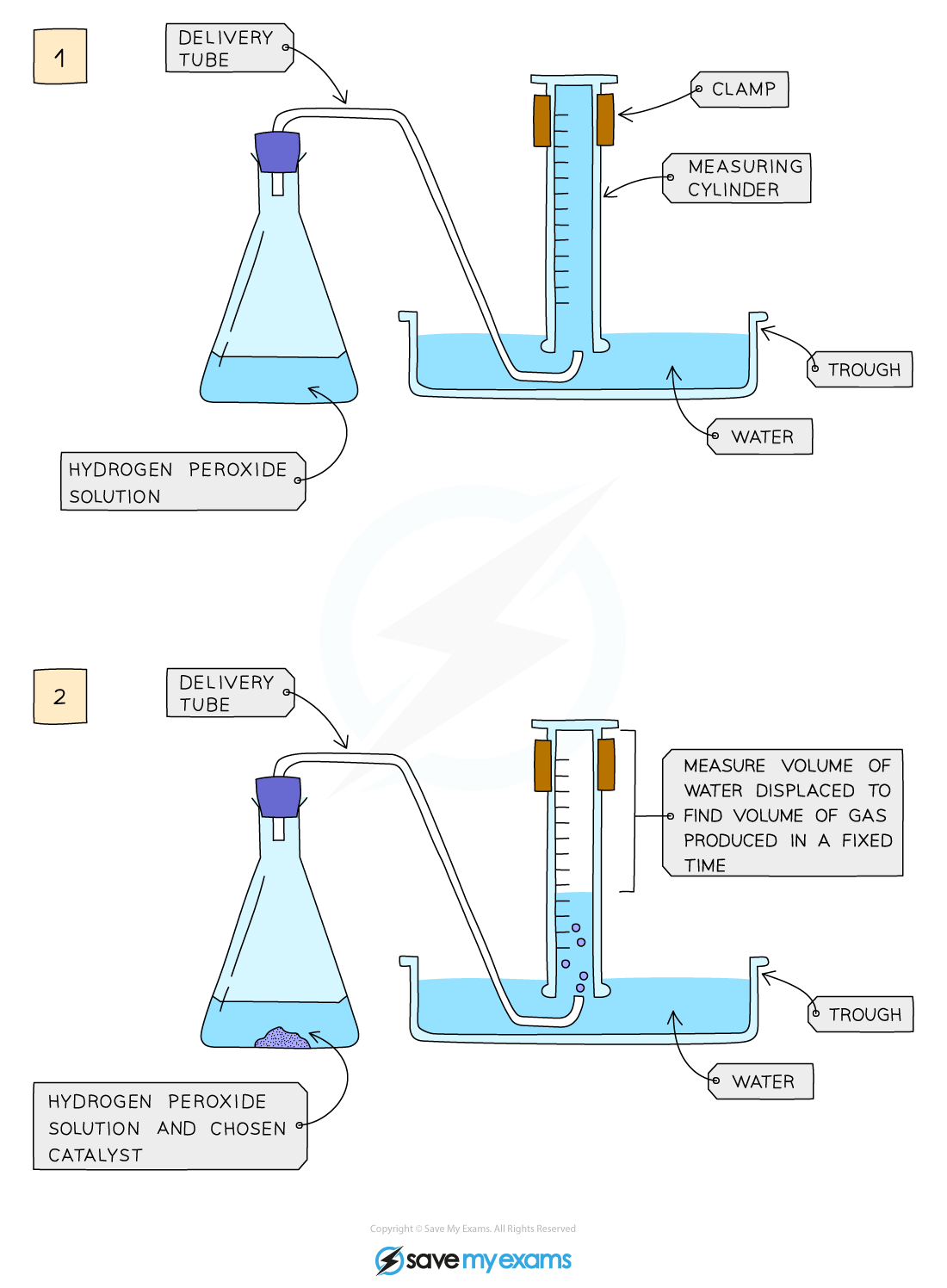
Edexcel IGCSE Chemistry 复习笔记 3.2.6 Practical Effect of Catalysts on
Testing For Rate Of Reaction To measure the rate of a reaction, we need to be able to measure either how quickly the reactants are used up or how quickly the products are formed. How do we monitor rates of reaction? Identify and apply factors affecting rates of. Explore the contrast between thermodynamics and kinetics,. Measuring the volume of gas given off by a reaction over time. Describe the rate of a reaction in terms of change of a measureable quantity, with time; There are three main methods of measuring rate: Learn how to measure reaction rates by monitoring the depletion of reactants or the formation of products. Find out how to calculate. Learn how to measure the rate of reaction by colour change using sodium thiosulfate and hydrochloric acid. Firstly, it’s important to understand what a rate of reaction is. To measure the rate of a reaction, we need to be able to measure either how quickly the reactants are used up or how quickly the products are formed.
From byjus.com
what is rate of reaction Testing For Rate Of Reaction Find out how to calculate. Learn how to measure the rate of reaction by colour change using sodium thiosulfate and hydrochloric acid. Identify and apply factors affecting rates of. Explore the contrast between thermodynamics and kinetics,. How do we monitor rates of reaction? Learn how to measure reaction rates by monitoring the depletion of reactants or the formation of products.. Testing For Rate Of Reaction.
From itnewstoday.net
The Best Way To Fix Error Sources For A Reaction Rate Lab IT News Today Testing For Rate Of Reaction There are three main methods of measuring rate: How do we monitor rates of reaction? Firstly, it’s important to understand what a rate of reaction is. Explore the contrast between thermodynamics and kinetics,. Describe the rate of a reaction in terms of change of a measureable quantity, with time; To measure the rate of a reaction, we need to be. Testing For Rate Of Reaction.
From postapplescientific.com
How To Measure the Rate of a Chemical Reaction Testing For Rate Of Reaction To measure the rate of a reaction, we need to be able to measure either how quickly the reactants are used up or how quickly the products are formed. How do we monitor rates of reaction? Find out how to calculate. Describe the rate of a reaction in terms of change of a measureable quantity, with time; Firstly, it’s important. Testing For Rate Of Reaction.
From byjus.com
What is the unit of rate constant for first order reaction Testing For Rate Of Reaction How do we monitor rates of reaction? Find out how to calculate. There are three main methods of measuring rate: Describe the rate of a reaction in terms of change of a measureable quantity, with time; Learn how to measure reaction rates by monitoring the depletion of reactants or the formation of products. Measuring the volume of gas given off. Testing For Rate Of Reaction.
From www.sasadoctor.com
Rate of reaction chemistry igcse Testing For Rate Of Reaction How do we monitor rates of reaction? Identify and apply factors affecting rates of. To measure the rate of a reaction, we need to be able to measure either how quickly the reactants are used up or how quickly the products are formed. Firstly, it’s important to understand what a rate of reaction is. Measuring the volume of gas given. Testing For Rate Of Reaction.
From www.tes.com
Measuring rates of reaction methods summary questions Teaching Resources Testing For Rate Of Reaction To measure the rate of a reaction, we need to be able to measure either how quickly the reactants are used up or how quickly the products are formed. Measuring the volume of gas given off by a reaction over time. How do we monitor rates of reaction? Find out how to calculate. Describe the rate of a reaction in. Testing For Rate Of Reaction.
From www.youtube.com
CTSC practical experiment Temperature & rate of reaction YouTube Testing For Rate Of Reaction There are three main methods of measuring rate: Find out how to calculate. Identify and apply factors affecting rates of. Measuring the volume of gas given off by a reaction over time. Firstly, it’s important to understand what a rate of reaction is. To measure the rate of a reaction, we need to be able to measure either how quickly. Testing For Rate Of Reaction.
From www.ctsc.org.za
FET Temperature and Rates of Reaction Experiment Cape Town Science Testing For Rate Of Reaction Describe the rate of a reaction in terms of change of a measureable quantity, with time; There are three main methods of measuring rate: Explore the contrast between thermodynamics and kinetics,. Learn how to measure the rate of reaction by colour change using sodium thiosulfate and hydrochloric acid. Firstly, it’s important to understand what a rate of reaction is. Measuring. Testing For Rate Of Reaction.
From www.slideshare.net
Rate of reaction Testing For Rate Of Reaction Firstly, it’s important to understand what a rate of reaction is. Learn how to measure the rate of reaction by colour change using sodium thiosulfate and hydrochloric acid. Learn how to measure reaction rates by monitoring the depletion of reactants or the formation of products. Find out how to calculate. There are three main methods of measuring rate: Measuring the. Testing For Rate Of Reaction.
From www.slideserve.com
PPT Rate of Reaction PowerPoint Presentation, free download ID2483456 Testing For Rate Of Reaction Find out how to calculate. How do we monitor rates of reaction? Measuring the volume of gas given off by a reaction over time. Identify and apply factors affecting rates of. There are three main methods of measuring rate: Describe the rate of a reaction in terms of change of a measureable quantity, with time; Explore the contrast between thermodynamics. Testing For Rate Of Reaction.
From wordwall.net
Rate of Reaction Quiz Testing For Rate Of Reaction Identify and apply factors affecting rates of. Firstly, it’s important to understand what a rate of reaction is. Learn how to measure reaction rates by monitoring the depletion of reactants or the formation of products. To measure the rate of a reaction, we need to be able to measure either how quickly the reactants are used up or how quickly. Testing For Rate Of Reaction.
From revisechemistry.uk
Rate of Reaction AQA C6 revisechemistry.uk Testing For Rate Of Reaction How do we monitor rates of reaction? Measuring the volume of gas given off by a reaction over time. To measure the rate of a reaction, we need to be able to measure either how quickly the reactants are used up or how quickly the products are formed. Learn how to measure reaction rates by monitoring the depletion of reactants. Testing For Rate Of Reaction.
From www.youtube.com
IGCSE Chemistry Rates of Reaction YouTube Testing For Rate Of Reaction Describe the rate of a reaction in terms of change of a measureable quantity, with time; Explore the contrast between thermodynamics and kinetics,. To measure the rate of a reaction, we need to be able to measure either how quickly the reactants are used up or how quickly the products are formed. There are three main methods of measuring rate:. Testing For Rate Of Reaction.
From sciencenotes.org
Factors That Affect Reaction Rate Chemical Testing For Rate Of Reaction There are three main methods of measuring rate: Find out how to calculate. Learn how to measure the rate of reaction by colour change using sodium thiosulfate and hydrochloric acid. Learn how to measure reaction rates by monitoring the depletion of reactants or the formation of products. Describe the rate of a reaction in terms of change of a measureable. Testing For Rate Of Reaction.
From www.youtube.com
Chemical 2 Average Rate of Reaction Instantaneous Rate Testing For Rate Of Reaction To measure the rate of a reaction, we need to be able to measure either how quickly the reactants are used up or how quickly the products are formed. Describe the rate of a reaction in terms of change of a measureable quantity, with time; Measuring the volume of gas given off by a reaction over time. Firstly, it’s important. Testing For Rate Of Reaction.
From www.youtube.com
Rate and Extent of Reaction Grade 12 Exam Questions YouTube Testing For Rate Of Reaction Identify and apply factors affecting rates of. Measuring the volume of gas given off by a reaction over time. Learn how to measure the rate of reaction by colour change using sodium thiosulfate and hydrochloric acid. How do we monitor rates of reaction? Explore the contrast between thermodynamics and kinetics,. There are three main methods of measuring rate: Describe the. Testing For Rate Of Reaction.
From www.chemicals.co.uk
How To Measure The Rate Of Reaction The Chemistry Blog Testing For Rate Of Reaction Learn how to measure the rate of reaction by colour change using sodium thiosulfate and hydrochloric acid. Describe the rate of a reaction in terms of change of a measureable quantity, with time; Firstly, it’s important to understand what a rate of reaction is. Measuring the volume of gas given off by a reaction over time. Find out how to. Testing For Rate Of Reaction.
From www.slideserve.com
PPT Part I Rates of Reaction PowerPoint Presentation, free Testing For Rate Of Reaction Learn how to measure reaction rates by monitoring the depletion of reactants or the formation of products. Describe the rate of a reaction in terms of change of a measureable quantity, with time; Firstly, it’s important to understand what a rate of reaction is. Measuring the volume of gas given off by a reaction over time. Explore the contrast between. Testing For Rate Of Reaction.
From webapi.bu.edu
💌 Factors that affect the reaction rate. Factors that Affect the Rate Testing For Rate Of Reaction Measuring the volume of gas given off by a reaction over time. There are three main methods of measuring rate: Identify and apply factors affecting rates of. Learn how to measure reaction rates by monitoring the depletion of reactants or the formation of products. Describe the rate of a reaction in terms of change of a measureable quantity, with time;. Testing For Rate Of Reaction.
From www.scribd.com
Rate of Reaction PDF Reaction Rate Chemical Reactions Testing For Rate Of Reaction Explore the contrast between thermodynamics and kinetics,. Describe the rate of a reaction in terms of change of a measureable quantity, with time; How do we monitor rates of reaction? Find out how to calculate. Learn how to measure the rate of reaction by colour change using sodium thiosulfate and hydrochloric acid. Identify and apply factors affecting rates of. Learn. Testing For Rate Of Reaction.
From www.youtube.com
Rate of Reaction and Rate Law Chemistry Class 12 IIT JEE Main Testing For Rate Of Reaction How do we monitor rates of reaction? Measuring the volume of gas given off by a reaction over time. Find out how to calculate. Learn how to measure the rate of reaction by colour change using sodium thiosulfate and hydrochloric acid. There are three main methods of measuring rate: To measure the rate of a reaction, we need to be. Testing For Rate Of Reaction.
From www.nagwa.com
Question Video Identifying Which Unit Is Not Commonly Used to Measure Testing For Rate Of Reaction Firstly, it’s important to understand what a rate of reaction is. Explore the contrast between thermodynamics and kinetics,. Describe the rate of a reaction in terms of change of a measureable quantity, with time; Identify and apply factors affecting rates of. How do we monitor rates of reaction? Find out how to calculate. There are three main methods of measuring. Testing For Rate Of Reaction.
From www.sasadoctor.com
Rate of reaction chemistry igcse Testing For Rate Of Reaction Explore the contrast between thermodynamics and kinetics,. Firstly, it’s important to understand what a rate of reaction is. Learn how to measure reaction rates by monitoring the depletion of reactants or the formation of products. Find out how to calculate. How do we monitor rates of reaction? To measure the rate of a reaction, we need to be able to. Testing For Rate Of Reaction.
From www.slideserve.com
PPT The rate of reaction PowerPoint Presentation, free download ID Testing For Rate Of Reaction Measuring the volume of gas given off by a reaction over time. There are three main methods of measuring rate: Firstly, it’s important to understand what a rate of reaction is. Describe the rate of a reaction in terms of change of a measureable quantity, with time; Learn how to measure reaction rates by monitoring the depletion of reactants or. Testing For Rate Of Reaction.
From www.studocu.com
Rates of Reaction Experiment 3a Diagram Method You will use the Testing For Rate Of Reaction To measure the rate of a reaction, we need to be able to measure either how quickly the reactants are used up or how quickly the products are formed. How do we monitor rates of reaction? Explore the contrast between thermodynamics and kinetics,. Learn how to measure the rate of reaction by colour change using sodium thiosulfate and hydrochloric acid.. Testing For Rate Of Reaction.
From www.chemistrylearner.com
Reaction Rate Definition, Formula, And Factors Affecting it Testing For Rate Of Reaction Learn how to measure the rate of reaction by colour change using sodium thiosulfate and hydrochloric acid. Learn how to measure reaction rates by monitoring the depletion of reactants or the formation of products. Find out how to calculate. To measure the rate of a reaction, we need to be able to measure either how quickly the reactants are used. Testing For Rate Of Reaction.
From www.sasadoctor.com
Rate of reaction chemistry igcse Testing For Rate Of Reaction Describe the rate of a reaction in terms of change of a measureable quantity, with time; Identify and apply factors affecting rates of. There are three main methods of measuring rate: Firstly, it’s important to understand what a rate of reaction is. Learn how to measure the rate of reaction by colour change using sodium thiosulfate and hydrochloric acid. Explore. Testing For Rate Of Reaction.
From www.tes.com
Rates of Reaction GCSE Chemistry Teaching Resources Testing For Rate Of Reaction Learn how to measure the rate of reaction by colour change using sodium thiosulfate and hydrochloric acid. How do we monitor rates of reaction? Identify and apply factors affecting rates of. Describe the rate of a reaction in terms of change of a measureable quantity, with time; To measure the rate of a reaction, we need to be able to. Testing For Rate Of Reaction.
From studylib.net
Reaction rates CIE iGCSE 0620 PPQ Testing For Rate Of Reaction Identify and apply factors affecting rates of. Describe the rate of a reaction in terms of change of a measureable quantity, with time; To measure the rate of a reaction, we need to be able to measure either how quickly the reactants are used up or how quickly the products are formed. How do we monitor rates of reaction? Learn. Testing For Rate Of Reaction.
From www.nagwa.com
Lesson Video Rates of Reactions Nagwa Testing For Rate Of Reaction Identify and apply factors affecting rates of. How do we monitor rates of reaction? To measure the rate of a reaction, we need to be able to measure either how quickly the reactants are used up or how quickly the products are formed. Firstly, it’s important to understand what a rate of reaction is. Explore the contrast between thermodynamics and. Testing For Rate Of Reaction.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 3.2.6 Practical Effect of Catalysts on Testing For Rate Of Reaction Learn how to measure the rate of reaction by colour change using sodium thiosulfate and hydrochloric acid. Describe the rate of a reaction in terms of change of a measureable quantity, with time; Learn how to measure reaction rates by monitoring the depletion of reactants or the formation of products. How do we monitor rates of reaction? Firstly, it’s important. Testing For Rate Of Reaction.
From www.sasadoctor.com
Rate of reaction chemistry igcse Testing For Rate Of Reaction Find out how to calculate. How do we monitor rates of reaction? To measure the rate of a reaction, we need to be able to measure either how quickly the reactants are used up or how quickly the products are formed. Measuring the volume of gas given off by a reaction over time. Learn how to measure the rate of. Testing For Rate Of Reaction.
From www.chegg.com
Solved Average Reaction Rate the change in reactant and Testing For Rate Of Reaction Explore the contrast between thermodynamics and kinetics,. To measure the rate of a reaction, we need to be able to measure either how quickly the reactants are used up or how quickly the products are formed. Firstly, it’s important to understand what a rate of reaction is. Describe the rate of a reaction in terms of change of a measureable. Testing For Rate Of Reaction.
From www.slideserve.com
PPT Title Calculating Rate of Reaction LI Calculate average rate of Testing For Rate Of Reaction Learn how to measure the rate of reaction by colour change using sodium thiosulfate and hydrochloric acid. There are three main methods of measuring rate: Learn how to measure reaction rates by monitoring the depletion of reactants or the formation of products. Describe the rate of a reaction in terms of change of a measureable quantity, with time; To measure. Testing For Rate Of Reaction.
From www.youtube.com
Calculating Rates of Reaction (2016) IB Biology YouTube Testing For Rate Of Reaction Learn how to measure reaction rates by monitoring the depletion of reactants or the formation of products. Find out how to calculate. How do we monitor rates of reaction? Explore the contrast between thermodynamics and kinetics,. Firstly, it’s important to understand what a rate of reaction is. There are three main methods of measuring rate: To measure the rate of. Testing For Rate Of Reaction.