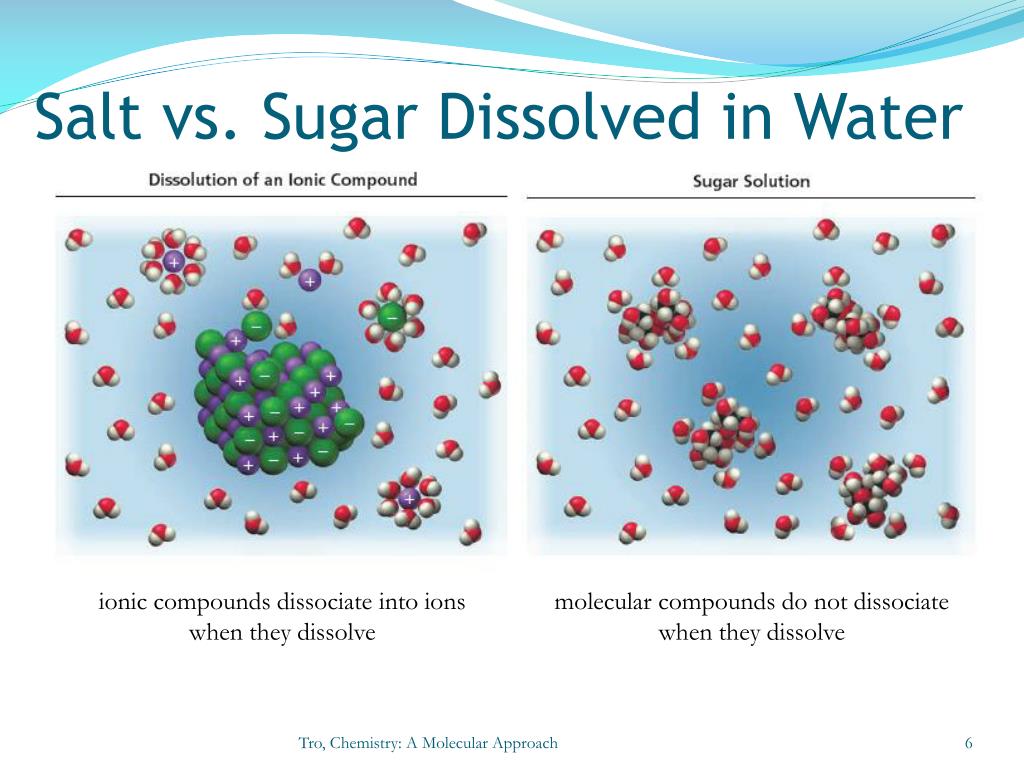
How Does Salt Dissolve Differently Than Sugar . As a demonstration, have students add salt, sugar, and alum to dirty water to show that alum cleans water more effectively than. Salt is made from positively charged sodium ions (gray) and negatively charged chloride ions (green). The sugar molecules move as whole molecules through the water. Your dissolving test would not be. What happens when sugar and salt are added to water? And, when you add 30 spoons into water the sugar just starts to stay in the bottom of The salt molecules break up into charged ions. Salt is about 25% more dense than sugar. Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and. Therefore, a teaspoon of salt weighs more than a teaspoon of sugar by almost 25%. Sugar can dissolve easier than salt because when you add 8 spoons of salt into water the salt already starts to stay in the bottom of the cup. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt:
from www.slideserve.com
Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: As a demonstration, have students add salt, sugar, and alum to dirty water to show that alum cleans water more effectively than. Your dissolving test would not be. Therefore, a teaspoon of salt weighs more than a teaspoon of sugar by almost 25%. And, when you add 30 spoons into water the sugar just starts to stay in the bottom of Salt is made from positively charged sodium ions (gray) and negatively charged chloride ions (green). Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and. Salt is about 25% more dense than sugar. The sugar molecules move as whole molecules through the water. The salt molecules break up into charged ions.
PPT Chapter 4 PowerPoint Presentation, free download ID762021
How Does Salt Dissolve Differently Than Sugar Sugar can dissolve easier than salt because when you add 8 spoons of salt into water the salt already starts to stay in the bottom of the cup. Therefore, a teaspoon of salt weighs more than a teaspoon of sugar by almost 25%. The salt molecules break up into charged ions. Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: As a demonstration, have students add salt, sugar, and alum to dirty water to show that alum cleans water more effectively than. The sugar molecules move as whole molecules through the water. What happens when sugar and salt are added to water? Sugar can dissolve easier than salt because when you add 8 spoons of salt into water the salt already starts to stay in the bottom of the cup. Your dissolving test would not be. And, when you add 30 spoons into water the sugar just starts to stay in the bottom of Salt is about 25% more dense than sugar. Salt is made from positively charged sodium ions (gray) and negatively charged chloride ions (green).
From www.wikidoc.org
Solution wikidoc How Does Salt Dissolve Differently Than Sugar As a demonstration, have students add salt, sugar, and alum to dirty water to show that alum cleans water more effectively than. Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and. Salt is made from positively charged sodium ions (gray) and negatively charged chloride ions (green). And, when you add 30 spoons into. How Does Salt Dissolve Differently Than Sugar.
From www.vecteezy.com
Dissolving science experiment with sugar dissolve in water 3333021 How Does Salt Dissolve Differently Than Sugar And, when you add 30 spoons into water the sugar just starts to stay in the bottom of The sugar molecules move as whole molecules through the water. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Sugar can dissolve easier than salt because when you add 8 spoons of salt into water the salt. How Does Salt Dissolve Differently Than Sugar.
From www.youtube.com
table salt dissolves in water YouTube How Does Salt Dissolve Differently Than Sugar The salt molecules break up into charged ions. Sugar can dissolve easier than salt because when you add 8 spoons of salt into water the salt already starts to stay in the bottom of the cup. Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and. The sugar molecules move as whole molecules through. How Does Salt Dissolve Differently Than Sugar.
From fyoiujhkk.blob.core.windows.net
How Does Salt Dissolve Ice at Victoria Lea blog How Does Salt Dissolve Differently Than Sugar What happens when sugar and salt are added to water? The sugar molecules move as whole molecules through the water. Therefore, a teaspoon of salt weighs more than a teaspoon of sugar by almost 25%. Sugar can dissolve easier than salt because when you add 8 spoons of salt into water the salt already starts to stay in the bottom. How Does Salt Dissolve Differently Than Sugar.
From slideplayer.com
Q2 Draw a picture of a solution and label the parts. ppt download How Does Salt Dissolve Differently Than Sugar And, when you add 30 spoons into water the sugar just starts to stay in the bottom of The salt molecules break up into charged ions. As a demonstration, have students add salt, sugar, and alum to dirty water to show that alum cleans water more effectively than. Pour in sugar, shake in salt, and evaporate water to see the. How Does Salt Dissolve Differently Than Sugar.
From www.scienceabc.com
Why Does Sugar Disappear When It Dissolves In Water? » ScienceABC How Does Salt Dissolve Differently Than Sugar Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: As a demonstration, have students add salt, sugar, and alum to dirty water to show that alum cleans water more effectively than. Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and. Therefore, a teaspoon of salt weighs more than. How Does Salt Dissolve Differently Than Sugar.
From studylibstearine.z21.web.core.windows.net
How Does Water Dissolve Salt How Does Salt Dissolve Differently Than Sugar Therefore, a teaspoon of salt weighs more than a teaspoon of sugar by almost 25%. The salt molecules break up into charged ions. And, when you add 30 spoons into water the sugar just starts to stay in the bottom of Your dissolving test would not be. Sugar can dissolve easier than salt because when you add 8 spoons of. How Does Salt Dissolve Differently Than Sugar.
From www.youtube.com
How Salt Dissolves in Water? YouTube How Does Salt Dissolve Differently Than Sugar Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: As a demonstration, have students add salt, sugar, and alum to dirty water to show that alum cleans water more effectively than. Sugar can dissolve easier than salt because when you add 8 spoons of salt into water the salt already starts to stay in the. How Does Salt Dissolve Differently Than Sugar.
From www.epicnaturalhealth.com
Does Sugar Dissolve In Oil? Epic Natural Health How Does Salt Dissolve Differently Than Sugar Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and. Salt is about 25% more dense than sugar. The salt molecules break up into charged ions. Therefore, a teaspoon of salt weighs more than a teaspoon of sugar by almost 25%. And, when you add 30 spoons into water the sugar just starts to. How Does Salt Dissolve Differently Than Sugar.
From www.slideserve.com
PPT Chemical Reactions Chapter 7 PowerPoint Presentation, free How Does Salt Dissolve Differently Than Sugar What happens when sugar and salt are added to water? Your dissolving test would not be. Salt is made from positively charged sodium ions (gray) and negatively charged chloride ions (green). Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Salt is about 25% more dense than sugar. The salt molecules break up into charged. How Does Salt Dissolve Differently Than Sugar.
From musicbykatie.com
Does Sugar Melt Or Dissolve? Trust The Answer How Does Salt Dissolve Differently Than Sugar Salt is made from positively charged sodium ions (gray) and negatively charged chloride ions (green). And, when you add 30 spoons into water the sugar just starts to stay in the bottom of As a demonstration, have students add salt, sugar, and alum to dirty water to show that alum cleans water more effectively than. The salt molecules break up. How Does Salt Dissolve Differently Than Sugar.
From www.koyuncusalt.com
Why Does Salt Dissolve In Water? How to Separate Them Back? Salt How Does Salt Dissolve Differently Than Sugar Sugar can dissolve easier than salt because when you add 8 spoons of salt into water the salt already starts to stay in the bottom of the cup. The salt molecules break up into charged ions. Salt is made from positively charged sodium ions (gray) and negatively charged chloride ions (green). Salt is about 25% more dense than sugar. Sugar. How Does Salt Dissolve Differently Than Sugar.
From mungfali.com
Dissolving Salt In Water Diagram How Does Salt Dissolve Differently Than Sugar Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and. The salt molecules break up into charged ions. The sugar molecules move as whole molecules through the water. Your dissolving test would not be. As a demonstration, have students add salt, sugar, and alum to dirty water to show that alum cleans water more. How Does Salt Dissolve Differently Than Sugar.
From www.sciencetimes.com
Does Adding Salt to Drinking Water Boost Hydration and Replenish How Does Salt Dissolve Differently Than Sugar And, when you add 30 spoons into water the sugar just starts to stay in the bottom of Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and. Salt is made from positively charged sodium ions (gray) and negatively charged chloride ions (green). The salt molecules break up into charged ions. Salt is about. How Does Salt Dissolve Differently Than Sugar.
From fyoiujhkk.blob.core.windows.net
How Does Salt Dissolve Ice at Victoria Lea blog How Does Salt Dissolve Differently Than Sugar Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and. The salt molecules break up into charged ions. Salt is made from positively charged sodium ions (gray) and negatively charged chloride ions (green). Your dissolving test would not be. And,. How Does Salt Dissolve Differently Than Sugar.
From www.youtube.com
Why Does This Powder Only Dissolve In Cold Water? YouTube How Does Salt Dissolve Differently Than Sugar Salt is about 25% more dense than sugar. The sugar molecules move as whole molecules through the water. And, when you add 30 spoons into water the sugar just starts to stay in the bottom of Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and. The salt molecules break up into charged ions.. How Does Salt Dissolve Differently Than Sugar.
From fyoiujhkk.blob.core.windows.net
How Does Salt Dissolve Ice at Victoria Lea blog How Does Salt Dissolve Differently Than Sugar What happens when sugar and salt are added to water? Sugar can dissolve easier than salt because when you add 8 spoons of salt into water the salt already starts to stay in the bottom of the cup. Salt is about 25% more dense than sugar. And, when you add 30 spoons into water the sugar just starts to stay. How Does Salt Dissolve Differently Than Sugar.
From www.science-sparks.com
Fun preschool science Making Mixtures How Does Salt Dissolve Differently Than Sugar Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and. What happens when sugar and salt are added to water? And, when you add 30 spoons into water the sugar just starts to stay in the bottom of Your dissolving test would not be. As a demonstration, have students add salt, sugar, and alum. How Does Salt Dissolve Differently Than Sugar.
From yesdirt.com
Does Sugar Dissolve in Alcohol? (ANSWERED) Yes Dirt How Does Salt Dissolve Differently Than Sugar Salt is about 25% more dense than sugar. Salt is made from positively charged sodium ions (gray) and negatively charged chloride ions (green). The salt molecules break up into charged ions. The sugar molecules move as whole molecules through the water. Therefore, a teaspoon of salt weighs more than a teaspoon of sugar by almost 25%. What happens when sugar. How Does Salt Dissolve Differently Than Sugar.
From www.acs.org
Lesson 5.6 Does Temperature Affect Dissolving? American Chemical Society How Does Salt Dissolve Differently Than Sugar Salt is about 25% more dense than sugar. Therefore, a teaspoon of salt weighs more than a teaspoon of sugar by almost 25%. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: The sugar molecules move as whole molecules through the water. The salt molecules break up into charged ions. Salt is made from positively. How Does Salt Dissolve Differently Than Sugar.
From www.vectorstock.com
How does sodium chloride nacl dissolve in water Vector Image How Does Salt Dissolve Differently Than Sugar The sugar molecules move as whole molecules through the water. Salt is made from positively charged sodium ions (gray) and negatively charged chloride ions (green). Sugar can dissolve easier than salt because when you add 8 spoons of salt into water the salt already starts to stay in the bottom of the cup. And, when you add 30 spoons into. How Does Salt Dissolve Differently Than Sugar.
From driverlayer.com
dissolve DriverLayer Search Engine How Does Salt Dissolve Differently Than Sugar Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and. The salt molecules break up into charged ions. Your dissolving test would not be. Salt is made from positively charged sodium ions (gray) and negatively charged chloride ions (green). Salt is about 25% more dense than sugar. Sugar can dissolve easier than salt because. How Does Salt Dissolve Differently Than Sugar.
From fyoiujhkk.blob.core.windows.net
How Does Salt Dissolve Ice at Victoria Lea blog How Does Salt Dissolve Differently Than Sugar Therefore, a teaspoon of salt weighs more than a teaspoon of sugar by almost 25%. Your dissolving test would not be. The sugar molecules move as whole molecules through the water. Salt is made from positively charged sodium ions (gray) and negatively charged chloride ions (green). Pour in sugar, shake in salt, and evaporate water to see the effects on. How Does Salt Dissolve Differently Than Sugar.
From fyoiujhkk.blob.core.windows.net
How Does Salt Dissolve Ice at Victoria Lea blog How Does Salt Dissolve Differently Than Sugar As a demonstration, have students add salt, sugar, and alum to dirty water to show that alum cleans water more effectively than. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: And, when you add 30 spoons into water the sugar just starts to stay in the bottom of Pour in sugar, shake in salt,. How Does Salt Dissolve Differently Than Sugar.
From eduvik.in
Matter in Our Surroundings Class 9 Notes Science Chapter 1 Eduvik How Does Salt Dissolve Differently Than Sugar Therefore, a teaspoon of salt weighs more than a teaspoon of sugar by almost 25%. The sugar molecules move as whole molecules through the water. Sugar can dissolve easier than salt because when you add 8 spoons of salt into water the salt already starts to stay in the bottom of the cup. What happens when sugar and salt are. How Does Salt Dissolve Differently Than Sugar.
From www.vecteezy.com
Dissolving science experiment with sugar dissolve in water 3188660 How Does Salt Dissolve Differently Than Sugar The salt molecules break up into charged ions. What happens when sugar and salt are added to water? The sugar molecules move as whole molecules through the water. Sugar can dissolve easier than salt because when you add 8 spoons of salt into water the salt already starts to stay in the bottom of the cup. Salt is about 25%. How Does Salt Dissolve Differently Than Sugar.
From www.twinkl.fr
What is Dissolving? Answered Twinkl Teaching Wiki How Does Salt Dissolve Differently Than Sugar Therefore, a teaspoon of salt weighs more than a teaspoon of sugar by almost 25%. Sugar can dissolve easier than salt because when you add 8 spoons of salt into water the salt already starts to stay in the bottom of the cup. The salt molecules break up into charged ions. Salt is made from positively charged sodium ions (gray). How Does Salt Dissolve Differently Than Sugar.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID762021 How Does Salt Dissolve Differently Than Sugar Therefore, a teaspoon of salt weighs more than a teaspoon of sugar by almost 25%. The sugar molecules move as whole molecules through the water. What happens when sugar and salt are added to water? The salt molecules break up into charged ions. Sugar can dissolve easier than salt because when you add 8 spoons of salt into water the. How Does Salt Dissolve Differently Than Sugar.
From byjus.com
Perform an activity to find out how to dissolve a solid in a liquid? How Does Salt Dissolve Differently Than Sugar Salt is about 25% more dense than sugar. Therefore, a teaspoon of salt weighs more than a teaspoon of sugar by almost 25%. Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and. The salt molecules break up into charged ions. The sugar molecules move as whole molecules through the water. Your dissolving test. How Does Salt Dissolve Differently Than Sugar.
From www.koyuncusalt.com
Why Does Salt Dissolve In Water? How to Separate Them Back? Salt How Does Salt Dissolve Differently Than Sugar Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and. The salt molecules break up into charged ions. Sugar can dissolve easier than salt because when you add 8 spoons of salt into water the salt already starts to stay in the bottom of the cup. And, when you add 30 spoons into water. How Does Salt Dissolve Differently Than Sugar.
From www.pinterest.com
Is Dissolving Salt in Water a Chemical Change or a Physical Change? How Does Salt Dissolve Differently Than Sugar What happens when sugar and salt are added to water? Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and. Sugar can dissolve easier than salt because when you add 8 spoons of salt into water the salt already starts to stay in the bottom of the cup. Therefore, a teaspoon of salt weighs. How Does Salt Dissolve Differently Than Sugar.
From wiringdiagrambig.z19.web.core.windows.net
Diagram Of Sugar Dissolving In Water How Does Salt Dissolve Differently Than Sugar Salt is about 25% more dense than sugar. And, when you add 30 spoons into water the sugar just starts to stay in the bottom of Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Sugar can dissolve easier than salt because when you add 8 spoons of salt into water the salt already starts. How Does Salt Dissolve Differently Than Sugar.
From saltsmart.org
How Does Salt Melt Snow and Ice? Salt Smart Collaborative How Does Salt Dissolve Differently Than Sugar And, when you add 30 spoons into water the sugar just starts to stay in the bottom of Therefore, a teaspoon of salt weighs more than a teaspoon of sugar by almost 25%. The sugar molecules move as whole molecules through the water. As a demonstration, have students add salt, sugar, and alum to dirty water to show that alum. How Does Salt Dissolve Differently Than Sugar.
From byjus.com
Why sugar dissolves faster in the water when stirred? How Does Salt Dissolve Differently Than Sugar Salt is about 25% more dense than sugar. Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Sugar can dissolve easier than salt because when you add 8 spoons of salt into water the salt already starts to stay. How Does Salt Dissolve Differently Than Sugar.
From howgem.com
Why Does Salt Melt Ice Faster Than Sugar How Gem How Does Salt Dissolve Differently Than Sugar The sugar molecules move as whole molecules through the water. Salt is about 25% more dense than sugar. Your dissolving test would not be. As a demonstration, have students add salt, sugar, and alum to dirty water to show that alum cleans water more effectively than. Pour in sugar, shake in salt, and evaporate water to see the effects on. How Does Salt Dissolve Differently Than Sugar.