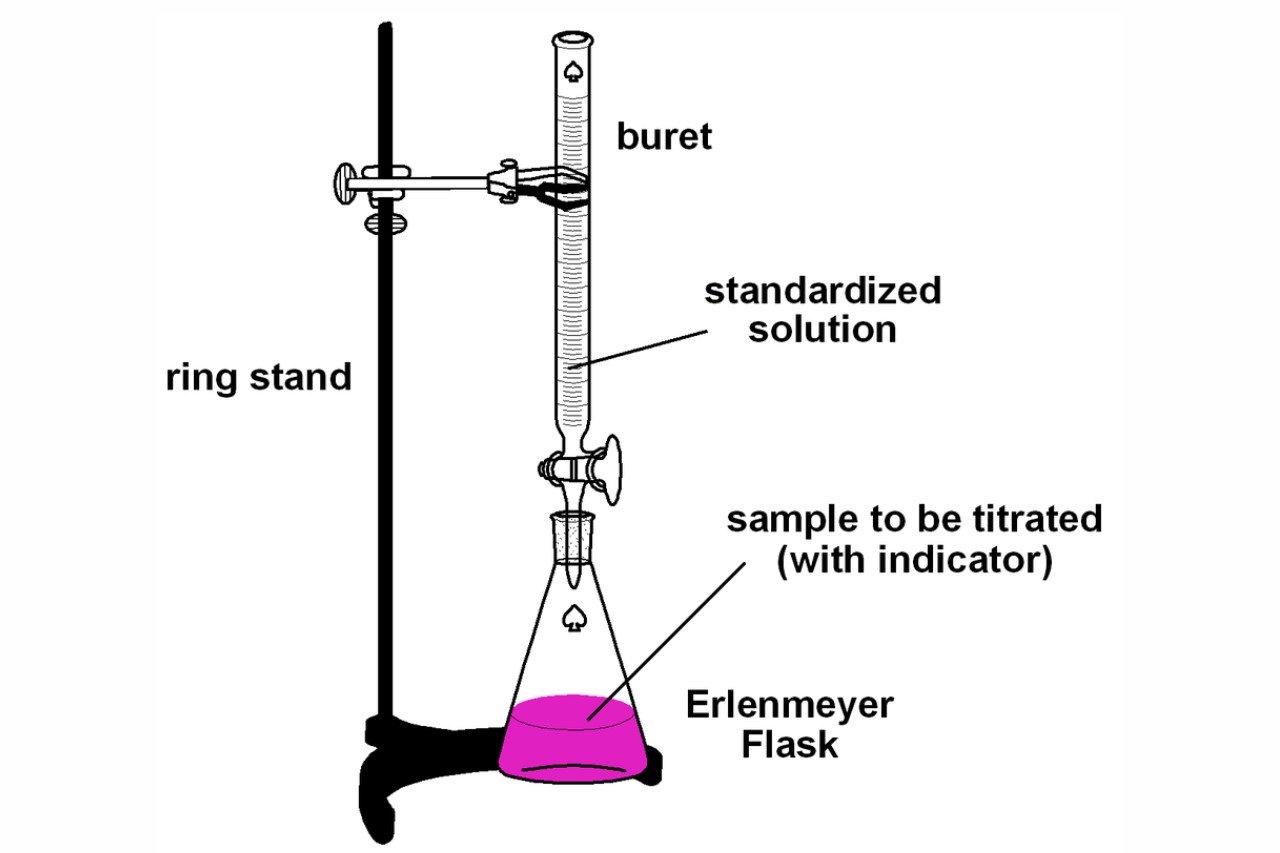
Titration Diagram Explained . Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. A chemical reaction is set up between a known volume of a solution of. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. It is based on the neutralization reaction, where an acid and a base react to form water and a salt.
from facts.net
Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. A chemical reaction is set up between a known volume of a solution of. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the.
8 Captivating Facts About AcidBase Titration
Titration Diagram Explained Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. A chemical reaction is set up between a known volume of a solution of. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Diagram Explained Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. A chemical reaction is set up between a known volume of a solution of. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. In. Titration Diagram Explained.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Diagram Explained It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known. Titration Diagram Explained.
From cwsimons.com
How to Draw Titration Curves of Amino Acids Food Science Toolbox Titration Diagram Explained Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid. Titration is a procedure used in chemistry in order to determine the molarity of an. Titration Diagram Explained.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Diagram Explained A chemical reaction is set up between a known volume of a solution of. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. It is based on the neutralization reaction, where an acid and a base react to form water and a. Titration Diagram Explained.
From ar.inspiredpencil.com
Titration Setup Diagram Titration Diagram Explained It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is the slow addition of one solution of a known concentration (called. Titration Diagram Explained.
From mmerevise.co.uk
pH Curves Questions and Revision MME Titration Diagram Explained Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. In an indicator based titration you add another chemical that changes color at. Titration Diagram Explained.
From www.shutterstock.com
Titration Setup Educational Chemistry Diagram Stock Illustration Titration Diagram Explained Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid. Learn about the titration technique through a practical experiment to. Titration Diagram Explained.
From ar.inspiredpencil.com
Titration Diagram Titration Diagram Explained It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and. Titration Diagram Explained.
From ar.inspiredpencil.com
Titration Diagram Titration Diagram Explained Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is a procedure used in chemistry in order to determine. Titration Diagram Explained.
From ar.inspiredpencil.com
Titration Diagram Titration Diagram Explained Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. It is based on the neutralization reaction, where an acid and a base react to form water and. Titration Diagram Explained.
From ar.inspiredpencil.com
Diagram Of Acid Base Titration Titration Diagram Explained It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. Titration Diagram Explained.
From ar.inspiredpencil.com
Titration Setup Diagram Titration Diagram Explained It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a. Titration Diagram Explained.
From facts.net
8 Captivating Facts About AcidBase Titration Titration Diagram Explained It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Learn about the titration technique through a practical experiment to determine the reacting. Titration Diagram Explained.
From proper-cooking.info
Titration Flask Diagram Titration Diagram Explained Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A chemical reaction is set up between a known volume of a solution of. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration involves the gradual. Titration Diagram Explained.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Diagram Explained It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known. Titration Diagram Explained.
From ar.inspiredpencil.com
Titration Diagram Titration Diagram Explained Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution. Titration Diagram Explained.
From ar.inspiredpencil.com
Titration Diagram Titration Diagram Explained Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. A chemical reaction is set up between a known volume. Titration Diagram Explained.
From owlcation.com
What Is Titration? The 3 Types of Titration Explained Owlcation Titration Diagram Explained Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A chemical reaction is set up between a known volume of a solution of. Titration involves the gradual. Titration Diagram Explained.
From ar.inspiredpencil.com
Titration Diagram Titration Diagram Explained Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid. Titration is a procedure used in chemistry in order to. Titration Diagram Explained.
From www.researchgate.net
Karl Fischer titration method (sources [30], [31], [32]) Download Titration Diagram Explained A chemical reaction is set up between a known volume of a solution of. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. In an indicator based. Titration Diagram Explained.
From www.easybiologyclass.com
What is Titration Curve? How Do You Find pKa? easybiologyclass Titration Diagram Explained It is based on the neutralization reaction, where an acid and a base react to form water and a salt. A chemical reaction is set up between a known volume of a solution of. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Titration involves the gradual addition of a. Titration Diagram Explained.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Diagram Explained Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration is the slow addition of one solution of a known. Titration Diagram Explained.
From ar.inspiredpencil.com
Titration Setup Diagram Titration Diagram Explained In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. It is based on the neutralization reaction, where an acid and a base react. Titration Diagram Explained.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Titration Diagram Explained Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is a procedure used in chemistry in order to determine. Titration Diagram Explained.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Diagram Explained Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. In an indicator based titration you add another chemical that changes color at the. Titration Diagram Explained.
From www.youtube.com
Amino Acids Titration Curves Explained YouTube Titration Diagram Explained Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. In an indicator based titration you add another chemical that changes color at the ph equal to. Titration Diagram Explained.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school Titration Diagram Explained Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. A chemical reaction is set up between a known volume of a solution of. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. In. Titration Diagram Explained.
From proper-cooking.info
Titration Diagram Titration Diagram Explained A chemical reaction is set up between a known volume of a solution of. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as. Titration Diagram Explained.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Titration Diagram Explained Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration is a procedure used in chemistry in order to determine the molarity of an. Titration Diagram Explained.
From mavink.com
Titration Diagram Titration Diagram Explained A chemical reaction is set up between a known volume of a solution of. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. In an indicator. Titration Diagram Explained.
From ar.inspiredpencil.com
Titration Setup Diagram Titration Diagram Explained Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. A chemical reaction is set up between a known volume of a solution of. It. Titration Diagram Explained.
From ar.inspiredpencil.com
Titration Diagram Titration Diagram Explained It is based on the neutralization reaction, where an acid and a base react to form water and a salt. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid. Titration is a procedure used in chemistry in order to determine the molarity of an acid or. Titration Diagram Explained.
From proper-cooking.info
Titration Diagram Titration Diagram Explained Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. A chemical reaction is set up between a known volume of a solution of. Titration involves the gradual. Titration Diagram Explained.
From ar.inspiredpencil.com
Titration Diagram Titration Diagram Explained Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid. It is based on the neutralization reaction, where an acid and a base react. Titration Diagram Explained.
From www.coursehero.com
[Solved] image details Label the titration diagram with the appropriate Titration Diagram Explained Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. A chemical reaction is set up between a known volume of a solution of. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. It is based on. Titration Diagram Explained.