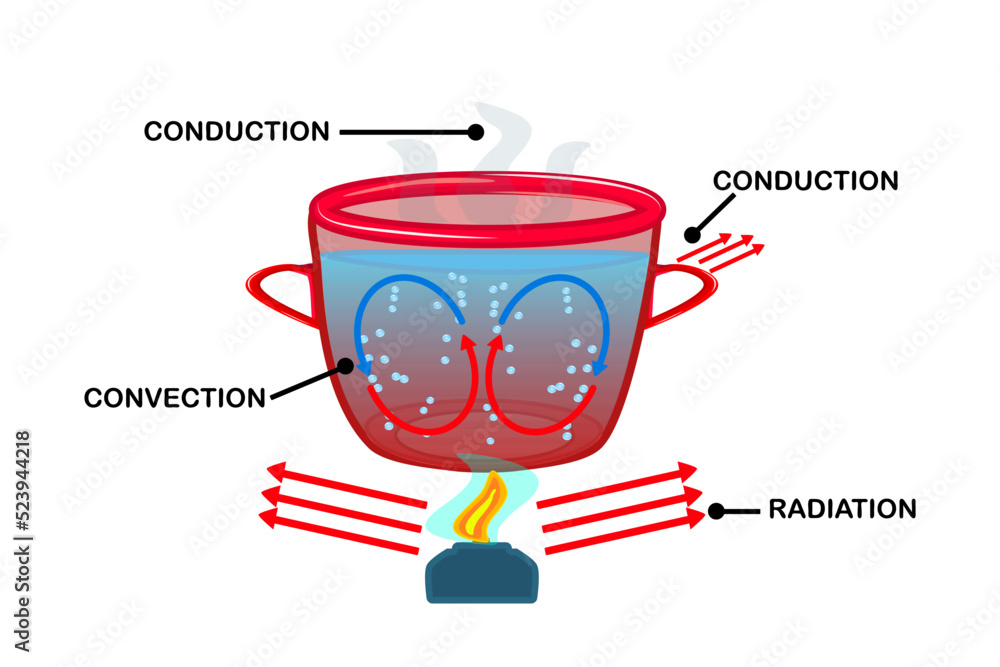
What Type Of Energy Is Boiling Water . Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. The change from a liquid phase to a gaseous phase. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. If you tried to raise liquid water above its boiling point, it would generate more entropy to convert the water to steam and store. Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. Fresh water freezes at 0° celsius. Phase changes in pure water occur at a specific temperature. At 1 atm, water freezes at 0° c and boils at 100° c. Hot air rises and cooler air sinks and replaces it.
from stock.adobe.com
If you tried to raise liquid water above its boiling point, it would generate more entropy to convert the water to steam and store. Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. Fresh water freezes at 0° celsius. The change from a liquid phase to a gaseous phase. Phase changes in pure water occur at a specific temperature. At 1 atm, water freezes at 0° c and boils at 100° c. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. Hot air rises and cooler air sinks and replaces it.
Vecteur Stock Heat transfer. Convection currents labeled diagram. Warm
What Type Of Energy Is Boiling Water Fresh water freezes at 0° celsius. Phase changes in pure water occur at a specific temperature. The change from a liquid phase to a gaseous phase. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Hot air rises and cooler air sinks and replaces it. Fresh water freezes at 0° celsius. At 1 atm, water freezes at 0° c and boils at 100° c. If you tried to raise liquid water above its boiling point, it would generate more entropy to convert the water to steam and store. Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules.
From uat.bpha.org.uk
Energy saving tips bpha What Type Of Energy Is Boiling Water Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. If you tried to raise liquid water above its boiling point, it would generate more entropy to convert the water to steam and store. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. The change from a. What Type Of Energy Is Boiling Water.
From www.youtube.com
Energy Stores and Transfers of a Kettle Boiling Water WORKED EXAMPLE What Type Of Energy Is Boiling Water Phase changes in pure water occur at a specific temperature. Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. Hot air rises and cooler air sinks and replaces it. Fresh water freezes at 0° celsius. At 1 atm, water freezes at 0° c and boils at 100°. What Type Of Energy Is Boiling Water.
From www.primalsurvivor.net
12 Ways to Boil Water Without Electricity What Type Of Energy Is Boiling Water Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. Phase changes in pure water occur at a specific temperature. Fresh water freezes at 0° celsius. Boiling is the process by which a. What Type Of Energy Is Boiling Water.
From jeopardylabs.com
6th Grade Thermal Energy Review By Mr. Gessert Jeopardy Template What Type Of Energy Is Boiling Water Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. At 1 atm, water freezes at 0° c and boils at 100° c. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. The change from a liquid phase to a gaseous phase.. What Type Of Energy Is Boiling Water.
From survivalstoic.com
Home Survival Stoic What Type Of Energy Is Boiling Water Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. Phase changes in pure water occur at a specific temperature. The change from a liquid phase to a gaseous phase. At 1 atm, water freezes at 0° c and boils at 100° c. Boiling is the process by which a liquid turns into a vapor. What Type Of Energy Is Boiling Water.
From stovesk.blogspot.com
How To Boil Water Without A Stove STOVESK What Type Of Energy Is Boiling Water Hot air rises and cooler air sinks and replaces it. Phase changes in pure water occur at a specific temperature. If you tried to raise liquid water above its boiling point, it would generate more entropy to convert the water to steam and store. Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential. What Type Of Energy Is Boiling Water.
From www.pinterest.com
The 2 types and 9 forms of Energy and Potential Physics What Type Of Energy Is Boiling Water Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. Hot air rises and cooler air sinks and replaces it. If you tried to raise liquid water above its boiling point, it would generate more entropy to convert the water to steam and store. The change from a liquid phase to a gaseous. What Type Of Energy Is Boiling Water.
From www.hardwareinterviews.fyi
Is it faster to cook pasta if you add it in before or after a pot of What Type Of Energy Is Boiling Water The change from a liquid phase to a gaseous phase. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. At 1 atm, water freezes at 0° c and boils at 100° c. If you tried to raise liquid water above its boiling point, it would generate more entropy to. What Type Of Energy Is Boiling Water.
From www.transformationtutoring.com
A sample of water is boiling as heat is added at a constant rate. Which What Type Of Energy Is Boiling Water At 1 atm, water freezes at 0° c and boils at 100° c. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. Phase changes in pure water occur at a specific temperature. Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy.. What Type Of Energy Is Boiling Water.
From www.youtube.com
What are Boiling Water Reactors? SkillLync YouTube What Type Of Energy Is Boiling Water At 1 atm, water freezes at 0° c and boils at 100° c. Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling water is an endothermic. What Type Of Energy Is Boiling Water.
From sciencenotes.org
What Are the Bubbles in Boiling Water? What Type Of Energy Is Boiling Water Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. The. What Type Of Energy Is Boiling Water.
From www.energy.gov
NUCLEAR 101 How Does a Nuclear Reactor Work? Department of Energy What Type Of Energy Is Boiling Water Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. If you tried to raise liquid water above its boiling point, it would generate more entropy to convert the water to steam and store. The change from a liquid phase to a gaseous phase. Phase changes in pure. What Type Of Energy Is Boiling Water.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock What Type Of Energy Is Boiling Water Hot air rises and cooler air sinks and replaces it. At 1 atm, water freezes at 0° c and boils at 100° c. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. Phase changes in pure water occur at a specific temperature. Boiling water is an endothermic process, which supplies heat to the water. What Type Of Energy Is Boiling Water.
From pressbooks.online.ucf.edu
Chapter 6, Heat The flow of energy and the direction of time What Type Of Energy Is Boiling Water If you tried to raise liquid water above its boiling point, it would generate more entropy to convert the water to steam and store. Phase changes in pure water occur at a specific temperature. At 1 atm, water freezes at 0° c and boils at 100° c. Fresh water freezes at 0° celsius. The change from a liquid phase to. What Type Of Energy Is Boiling Water.
From survivalstoic.com
How To Boil Water Without Electricity 15 Easy Ways What Type Of Energy Is Boiling Water Fresh water freezes at 0° celsius. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. Phase changes in pure water occur at a specific temperature. Boiling water is when water changes phase from a liquid to a. What Type Of Energy Is Boiling Water.
From phys.libretexts.org
1.4 Working With the EnergyInteraction Model Physics LibreTexts What Type Of Energy Is Boiling Water Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Fresh water freezes at 0° celsius. At 1 atm, water freezes at 0° c and boils at 100° c. Hot air rises and cooler air sinks and replaces it. Phase changes in pure water occur at a specific temperature. If. What Type Of Energy Is Boiling Water.
From www.dreamstime.com
Diagram Illustrating How Heat is Transferred in a Boiling Pot Stock What Type Of Energy Is Boiling Water The change from a liquid phase to a gaseous phase. Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Hot air rises and cooler air sinks and replaces it. Phase changes in. What Type Of Energy Is Boiling Water.
From knowyourmeme.com
On the Other Hand, It's Slowing Things Down, Too Electricity Is Just What Type Of Energy Is Boiling Water Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. Boiling is the process by which. What Type Of Energy Is Boiling Water.
From thepiquelab.com
Tackling Heat Energy Questions Melting & Boiling Points! Primary What Type Of Energy Is Boiling Water Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. If you tried to raise liquid water above its boiling point, it would generate more entropy to convert the water to steam and store. At 1 atm, water freezes at 0° c and boils at 100° c. Phase changes in pure water occur at a. What Type Of Energy Is Boiling Water.
From doomsdayden.com
How To Boil Water Without Electricity In 6 Easy Steps What Type Of Energy Is Boiling Water Phase changes in pure water occur at a specific temperature. Fresh water freezes at 0° celsius. Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. The change from a liquid phase to a gaseous phase. If you tried to raise liquid water above its boiling point, it. What Type Of Energy Is Boiling Water.
From stock.adobe.com
Vecteur Stock Heat transfer. Convection currents labeled diagram. Warm What Type Of Energy Is Boiling Water Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. Hot air rises and cooler air sinks and replaces it. If you tried to raise liquid water above its boiling point, it would generate more entropy to convert the water to steam and store. Boiling water is when water changes phase from a liquid to. What Type Of Energy Is Boiling Water.
From arenahanna.wordpress.com
Transfer processing of heat energy HEAT WORLD OF PHYSICS What Type Of Energy Is Boiling Water If you tried to raise liquid water above its boiling point, it would generate more entropy to convert the water to steam and store. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. The change from a liquid phase to a gaseous phase. Boiling water is when water changes phase from a liquid to. What Type Of Energy Is Boiling Water.
From letstalkscience.ca
Introduction to Heat Transfer Let's Talk Science What Type Of Energy Is Boiling Water Phase changes in pure water occur at a specific temperature. If you tried to raise liquid water above its boiling point, it would generate more entropy to convert the water to steam and store. Hot air rises and cooler air sinks and replaces it. Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential. What Type Of Energy Is Boiling Water.
From www.youtube.com
Introduction to Boiling Heat Transfer Boiling and Condensation Heat What Type Of Energy Is Boiling Water At 1 atm, water freezes at 0° c and boils at 100° c. If you tried to raise liquid water above its boiling point, it would generate more entropy to convert the water to steam and store. Hot air rises and cooler air sinks and replaces it. Boiling water is an endothermic process, which supplies heat to the water molecules,. What Type Of Energy Is Boiling Water.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Type Of Energy Is Boiling Water Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. At 1 atm, water freezes at 0° c and boils at 100° c. The change from a liquid phase to a gaseous phase. Phase changes in pure water occur at a specific temperature. Boiling water undergoes convection as less dense. What Type Of Energy Is Boiling Water.
From www.youtube.com
Energy required reach the boiling temperature of water or to reach 100 What Type Of Energy Is Boiling Water At 1 atm, water freezes at 0° c and boils at 100° c. Hot air rises and cooler air sinks and replaces it. Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules.. What Type Of Energy Is Boiling Water.
From www.gauthmath.com
Solved Which type of power station does not use steam from boiling What Type Of Energy Is Boiling Water Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. Phase changes in pure water occur at a specific temperature. The change from a liquid phase to a gaseous phase. Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. Boiling water is. What Type Of Energy Is Boiling Water.
From www.armstrongeconomics.com
Civil Unrest, Revolution, & The Phase Transition Curve Armstrong What Type Of Energy Is Boiling Water Hot air rises and cooler air sinks and replaces it. Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. Boiling is the process by which a liquid turns into a vapor when it is heated to its. What Type Of Energy Is Boiling Water.
From favpng.com
Boiling Point Melting Point Heat Temperature Chemistry, PNG What Type Of Energy Is Boiling Water If you tried to raise liquid water above its boiling point, it would generate more entropy to convert the water to steam and store. Phase changes in pure water occur at a specific temperature. Fresh water freezes at 0° celsius. Hot air rises and cooler air sinks and replaces it. Boiling water is an endothermic process, which supplies heat to. What Type Of Energy Is Boiling Water.
From teltruhome.com
Thermometer Accuracy What Type Of Energy Is Boiling Water Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. If you tried to raise liquid water above its boiling point, it would generate more entropy to convert the water to steam and store. Phase changes in pure water occur at a specific temperature. At 1 atm, water. What Type Of Energy Is Boiling Water.
From www.vecteezy.com
Diagram showing Methods of Heat Transfer 2790593 Vector Art at Vecteezy What Type Of Energy Is Boiling Water Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. Phase changes in pure water occur at a specific temperature. Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. At 1 atm, water freezes at 0° c and boils at 100° c.. What Type Of Energy Is Boiling Water.
From knowyourmeme.com
That's It? That Was the Nuclear Power? Electricity Is Just Boiling What Type Of Energy Is Boiling Water If you tried to raise liquid water above its boiling point, it would generate more entropy to convert the water to steam and store. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. At 1 atm, water freezes at 0° c and boils at 100° c. The change from a liquid phase to a. What Type Of Energy Is Boiling Water.
From bmxracingthailand.com
How To Heat Water Without Electricity Or Fire? New Update What Type Of Energy Is Boiling Water Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. Fresh water freezes at 0° celsius. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. The change from a liquid phase to a gaseous phase. If you tried to raise liquid water above its boiling point, it. What Type Of Energy Is Boiling Water.
From www.bartleby.com
Boiling Point of Water bartleby What Type Of Energy Is Boiling Water Fresh water freezes at 0° celsius. Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. If you tried to raise liquid water above its boiling point, it would generate more. What Type Of Energy Is Boiling Water.
From www.world-nuclear.org
Gallery World Nuclear Association What Type Of Energy Is Boiling Water Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. If you tried to raise liquid water above its boiling point, it would generate more entropy to convert the water to steam and store. Boiling is the process by which a liquid turns into a vapor when it. What Type Of Energy Is Boiling Water.