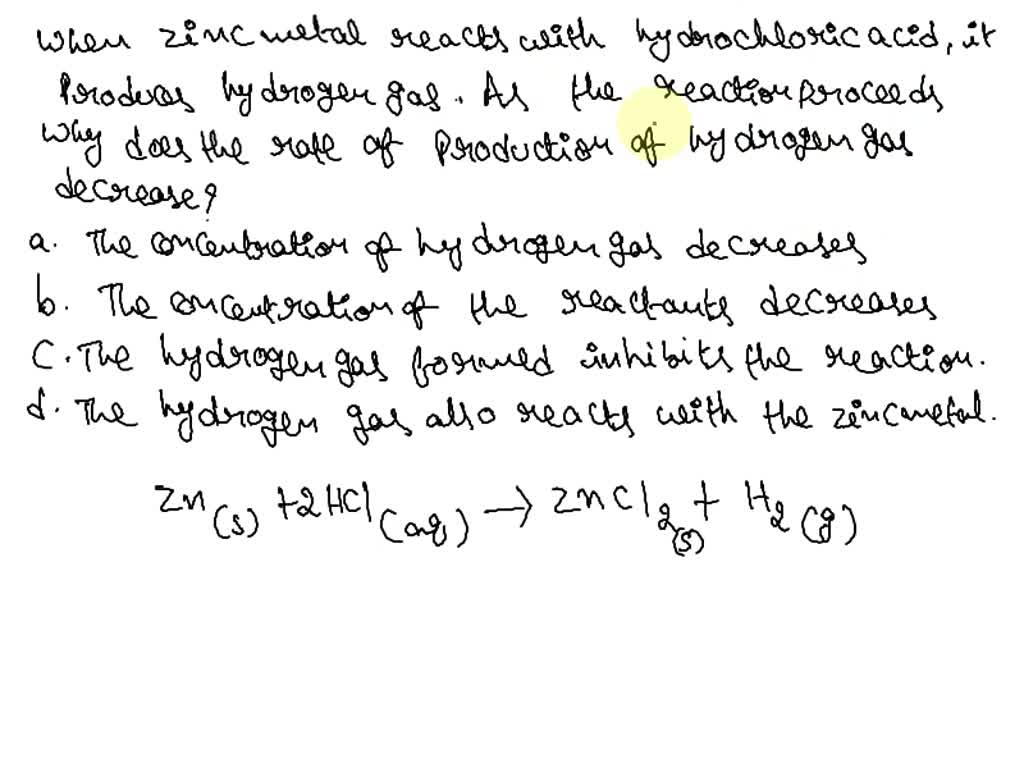Balancing Equation Zn Hcl Zncl2 H2 . Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. first, we set all coefficients to 1: 1 zn + 1 hcl = 1 h 2 + 1 zncl 2. Zn + hcl = zncl2 + h2. Solved and balanced chemical equation. balance the following equation:zn + hcl → zncl2 +. enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. For each element, we check if the number of atoms is balanced. A chemical equation in which the number of atoms of each element on both sides of. zinc metal reacts with hydrochloric acid according to the balanced equation: to balance the chemical equation zn + hcl = zncl2 + h2 you first.
from www.numerade.com
enter an equation of an ionic chemical equation and press the balance button. 1 zn + 1 hcl = 1 h 2 + 1 zncl 2. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. The balanced equation will be calculated along. Solved and balanced chemical equation. Zn + hcl = zncl2 + h2. balance the following equation:zn + hcl → zncl2 +. zinc metal reacts with hydrochloric acid according to the balanced equation: first, we set all coefficients to 1: A chemical equation in which the number of atoms of each element on both sides of.
SOLVED The equation below shows the reaction of zinc with hydrochloric
Balancing Equation Zn Hcl Zncl2 H2 Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. to balance the chemical equation zn + hcl = zncl2 + h2 you first. enter an equation of an ionic chemical equation and press the balance button. zinc metal reacts with hydrochloric acid according to the balanced equation: Solved and balanced chemical equation. balance the following equation:zn + hcl → zncl2 +. first, we set all coefficients to 1: Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. Zn + hcl = zncl2 + h2. 1 zn + 1 hcl = 1 h 2 + 1 zncl 2. A chemical equation in which the number of atoms of each element on both sides of. For each element, we check if the number of atoms is balanced. The balanced equation will be calculated along.
From www.numerade.com
SOLVED Balance each of these equations Li (s) + O2 (g) > Li2O (g Balancing Equation Zn Hcl Zncl2 H2 Solved and balanced chemical equation. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. The balanced equation will be calculated along. first, we set all coefficients to 1: zinc metal reacts with hydrochloric acid according to the balanced equation: enter an equation of an ionic chemical. Balancing Equation Zn Hcl Zncl2 H2.
From www.youtube.com
How to balance Zn + HCl = ZnCl2 + H2 YouTube Balancing Equation Zn Hcl Zncl2 H2 zinc metal reacts with hydrochloric acid according to the balanced equation: first, we set all coefficients to 1: enter an equation of an ionic chemical equation and press the balance button. to balance the chemical equation zn + hcl = zncl2 + h2 you first. For each element, we check if the number of atoms is. Balancing Equation Zn Hcl Zncl2 H2.
From brainly.in
3. Balance the following reactioni HCl+Zn=ZnCl2+H2 Brainly.in Balancing Equation Zn Hcl Zncl2 H2 balance the following equation:zn + hcl → zncl2 +. For each element, we check if the number of atoms is balanced. The balanced equation will be calculated along. zinc metal reacts with hydrochloric acid according to the balanced equation: Solved and balanced chemical equation. Zn + hcl = zncl2 + h2. Zn (s) + 2 hcl (aq)¡zncl2 (aq). Balancing Equation Zn Hcl Zncl2 H2.
From radioabierta.net
Zn + 2HCl → ZnCl2 + H2 Реакция цинка и соляной кислоты เนื้อหา Balancing Equation Zn Hcl Zncl2 H2 Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. balance the following equation:zn + hcl → zncl2 +. For each element, we check if the number of atoms is balanced. Solved and balanced chemical equation. A chemical equation in which the number of atoms of each element on. Balancing Equation Zn Hcl Zncl2 H2.
From www.slideserve.com
PPT Balancing Chemical Equations 2 PowerPoint Presentation, free Balancing Equation Zn Hcl Zncl2 H2 balance the following equation:zn + hcl → zncl2 +. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. to balance the chemical equation zn + hcl = zncl2 + h2 you first. Zn + hcl = zncl2 + h2. zinc metal reacts with hydrochloric acid according. Balancing Equation Zn Hcl Zncl2 H2.
From www.slideserve.com
PPT Balancing Equations Practice PowerPoint Presentation, free Balancing Equation Zn Hcl Zncl2 H2 1 zn + 1 hcl = 1 h 2 + 1 zncl 2. Solved and balanced chemical equation. enter an equation of an ionic chemical equation and press the balance button. Zn + hcl = zncl2 + h2. For each element, we check if the number of atoms is balanced. zinc metal reacts with hydrochloric acid according to. Balancing Equation Zn Hcl Zncl2 H2.
From www.youtube.com
How to balance Zn + Hcl = Zncl2 + H2 YouTube Balancing Equation Zn Hcl Zncl2 H2 first, we set all coefficients to 1: Zn + hcl = zncl2 + h2. The balanced equation will be calculated along. balance the following equation:zn + hcl → zncl2 +. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. For each element, we check if the number. Balancing Equation Zn Hcl Zncl2 H2.
From www.youtube.com
Balance the following chemical equation Zn + HCl → ZnCl2 + H2 YouTube Balancing Equation Zn Hcl Zncl2 H2 to balance the chemical equation zn + hcl = zncl2 + h2 you first. 1 zn + 1 hcl = 1 h 2 + 1 zncl 2. A chemical equation in which the number of atoms of each element on both sides of. balance the following equation:zn + hcl → zncl2 +. Zn + hcl = zncl2 +. Balancing Equation Zn Hcl Zncl2 H2.
From www.numerade.com
SOLVED Balance the reaction. A coefficient of "1" is understood Balancing Equation Zn Hcl Zncl2 H2 zinc metal reacts with hydrochloric acid according to the balanced equation: For each element, we check if the number of atoms is balanced. balance the following equation:zn + hcl → zncl2 +. enter an equation of an ionic chemical equation and press the balance button. first, we set all coefficients to 1: Zn + hcl =. Balancing Equation Zn Hcl Zncl2 H2.
From www.youtube.com
How to balance Zn + HCl → ZnCl2 + H2 YouTube Balancing Equation Zn Hcl Zncl2 H2 Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. to balance the chemical equation zn + hcl = zncl2 + h2 you first. 1 zn + 1 hcl = 1 h 2 + 1 zncl 2. The balanced equation will be calculated along. zinc metal reacts with. Balancing Equation Zn Hcl Zncl2 H2.
From www.numerade.com
SOLVED Stoichiometric Calculations Calculate the mass of hydrochloric Balancing Equation Zn Hcl Zncl2 H2 Zn + hcl = zncl2 + h2. enter an equation of an ionic chemical equation and press the balance button. Solved and balanced chemical equation. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. A chemical equation in which the number of atoms of each element on both. Balancing Equation Zn Hcl Zncl2 H2.
From www.youtube.com
How to Balance Zn + HCl = ZnCl2 + H2 YouTube Balancing Equation Zn Hcl Zncl2 H2 Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. zinc metal reacts with hydrochloric acid according to the balanced equation: first, we set all coefficients to 1: 1 zn + 1 hcl = 1 h 2 + 1 zncl 2. balance the following equation:zn + hcl. Balancing Equation Zn Hcl Zncl2 H2.
From byjus.com
10g of Zn reacts with 2g of hcl according to the equation.zn+hcl=Zncl2 Balancing Equation Zn Hcl Zncl2 H2 Solved and balanced chemical equation. to balance the chemical equation zn + hcl = zncl2 + h2 you first. zinc metal reacts with hydrochloric acid according to the balanced equation: The balanced equation will be calculated along. Zn + hcl = zncl2 + h2. For each element, we check if the number of atoms is balanced. A chemical. Balancing Equation Zn Hcl Zncl2 H2.
From www.youtube.com
How to balance Zn + HCl = ZnCl2 + H2 YouTube Balancing Equation Zn Hcl Zncl2 H2 enter an equation of an ionic chemical equation and press the balance button. Zn + hcl = zncl2 + h2. The balanced equation will be calculated along. zinc metal reacts with hydrochloric acid according to the balanced equation: Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with.. Balancing Equation Zn Hcl Zncl2 H2.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the Balancing Equation Zn Hcl Zncl2 H2 zinc metal reacts with hydrochloric acid according to the balanced equation: 1 zn + 1 hcl = 1 h 2 + 1 zncl 2. balance the following equation:zn + hcl → zncl2 +. enter an equation of an ionic chemical equation and press the balance button. Zn + hcl = zncl2 + h2. to balance the. Balancing Equation Zn Hcl Zncl2 H2.
From www.numerade.com
SOLVED Calculate the mass of hydrochloric acid (HCl) needed to react Balancing Equation Zn Hcl Zncl2 H2 zinc metal reacts with hydrochloric acid according to the balanced equation: The balanced equation will be calculated along. A chemical equation in which the number of atoms of each element on both sides of. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. balance the following equation:zn. Balancing Equation Zn Hcl Zncl2 H2.
From www.numerade.com
SOLVEDBalance each of these equations, and then write the complete Balancing Equation Zn Hcl Zncl2 H2 balance the following equation:zn + hcl → zncl2 +. to balance the chemical equation zn + hcl = zncl2 + h2 you first. The balanced equation will be calculated along. zinc metal reacts with hydrochloric acid according to the balanced equation: Zn + hcl = zncl2 + h2. Solved and balanced chemical equation. 1 zn + 1. Balancing Equation Zn Hcl Zncl2 H2.
From www.numerade.com
SOLVED Experiment 18 Chemical Reactions Lab Report Name Section Balancing Equation Zn Hcl Zncl2 H2 Solved and balanced chemical equation. For each element, we check if the number of atoms is balanced. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. to balance the chemical equation zn + hcl = zncl2 + h2 you first. enter an equation of an ionic chemical. Balancing Equation Zn Hcl Zncl2 H2.
From www.numerade.com
SOLVED Balance this chemical equation. Choose "blank" for the box if Balancing Equation Zn Hcl Zncl2 H2 1 zn + 1 hcl = 1 h 2 + 1 zncl 2. For each element, we check if the number of atoms is balanced. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. Zn + hcl = zncl2 + h2. first, we set all coefficients to 1:. Balancing Equation Zn Hcl Zncl2 H2.
From www.numerade.com
In this problem, zinc reacts with hydrochloric acid to make zinc Balancing Equation Zn Hcl Zncl2 H2 For each element, we check if the number of atoms is balanced. Solved and balanced chemical equation. balance the following equation:zn + hcl → zncl2 +. A chemical equation in which the number of atoms of each element on both sides of. zinc metal reacts with hydrochloric acid according to the balanced equation: Zn (s) + 2 hcl. Balancing Equation Zn Hcl Zncl2 H2.
From booksbibliophile2021.blogspot.com
Zn Hcl Zncl2 H2 Balance Equation 15+ Pages Solution [725kb] Updated Balancing Equation Zn Hcl Zncl2 H2 For each element, we check if the number of atoms is balanced. to balance the chemical equation zn + hcl = zncl2 + h2 you first. A chemical equation in which the number of atoms of each element on both sides of. 1 zn + 1 hcl = 1 h 2 + 1 zncl 2. balance the following. Balancing Equation Zn Hcl Zncl2 H2.
From www.youtube.com
Comment équilibrer ? Zn + HCl → ZnCl2 + H2 équation chimique YouTube Balancing Equation Zn Hcl Zncl2 H2 Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. Solved and balanced chemical equation. 1 zn + 1 hcl = 1 h 2 + 1 zncl 2. to balance the chemical equation zn + hcl = zncl2 + h2 you first. For each element, we check if the. Balancing Equation Zn Hcl Zncl2 H2.
From brainly.in
Balance the following equations Zn + HCl > ZnCl2 + H2 KClO3 > KCl Balancing Equation Zn Hcl Zncl2 H2 zinc metal reacts with hydrochloric acid according to the balanced equation: Zn + hcl = zncl2 + h2. The balanced equation will be calculated along. A chemical equation in which the number of atoms of each element on both sides of. balance the following equation:zn + hcl → zncl2 +. Solved and balanced chemical equation. first, we. Balancing Equation Zn Hcl Zncl2 H2.
From booksbibliophile2021.blogspot.com
Zn Hcl Zncl2 H2 Balance Equation 15+ Pages Solution [725kb] Updated Balancing Equation Zn Hcl Zncl2 H2 first, we set all coefficients to 1: enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. balance the following equation:zn + hcl → zncl2 +. For each element, we check if the number of atoms is balanced. to balance the chemical equation zn + hcl. Balancing Equation Zn Hcl Zncl2 H2.
From www.youtube.com
Balanced chemical equation balancing Zn+HCl 》ZnCl2 +H2 example for Balancing Equation Zn Hcl Zncl2 H2 Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. to balance the chemical equation zn + hcl = zncl2 + h2 you first. first, we set all coefficients to 1: zinc metal reacts with hydrochloric acid according to the balanced equation: balance the following equation:zn. Balancing Equation Zn Hcl Zncl2 H2.
From sciencenotes.org
Balancing Chemical Equations Practice Sheet Balancing Equation Zn Hcl Zncl2 H2 Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. For each element, we check if the number of atoms is balanced. Solved and balanced chemical equation. first, we set all coefficients to 1: 1 zn + 1 hcl = 1 h 2 + 1 zncl 2. The balanced. Balancing Equation Zn Hcl Zncl2 H2.
From www.slideserve.com
PPT REACTION PREDICTION PreAP CHEMISTRY PowerPoint Presentation Balancing Equation Zn Hcl Zncl2 H2 A chemical equation in which the number of atoms of each element on both sides of. For each element, we check if the number of atoms is balanced. The balanced equation will be calculated along. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. first, we set all. Balancing Equation Zn Hcl Zncl2 H2.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the Balancing Equation Zn Hcl Zncl2 H2 1 zn + 1 hcl = 1 h 2 + 1 zncl 2. first, we set all coefficients to 1: zinc metal reacts with hydrochloric acid according to the balanced equation: enter an equation of an ionic chemical equation and press the balance button. A chemical equation in which the number of atoms of each element on. Balancing Equation Zn Hcl Zncl2 H2.
From www.chegg.com
Solved 1. The reaction of zinc metal and hydrochloric acid Balancing Equation Zn Hcl Zncl2 H2 zinc metal reacts with hydrochloric acid according to the balanced equation: For each element, we check if the number of atoms is balanced. first, we set all coefficients to 1: to balance the chemical equation zn + hcl = zncl2 + h2 you first. Solved and balanced chemical equation. The balanced equation will be calculated along. 1. Balancing Equation Zn Hcl Zncl2 H2.
From www.youtube.com
Zn+HCl=ZnCl2+H2 balance the chemical equation mydocumentary838. zn+hcl Balancing Equation Zn Hcl Zncl2 H2 balance the following equation:zn + hcl → zncl2 +. to balance the chemical equation zn + hcl = zncl2 + h2 you first. The balanced equation will be calculated along. Solved and balanced chemical equation. For each element, we check if the number of atoms is balanced. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g). Balancing Equation Zn Hcl Zncl2 H2.
From www.numerade.com
SOLVED Balance the following equations and upload your solutions as a Balancing Equation Zn Hcl Zncl2 H2 to balance the chemical equation zn + hcl = zncl2 + h2 you first. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. first, we set all coefficients to 1: Zn + hcl = zncl2 + h2. balance the following equation:zn + hcl → zncl2 +.. Balancing Equation Zn Hcl Zncl2 H2.
From www.youtube.com
How to balance Zn+HCl=ZnCl2+H2Chemical equation Zn+HCl=ZnCl2+H2 Balancing Equation Zn Hcl Zncl2 H2 zinc metal reacts with hydrochloric acid according to the balanced equation: Solved and balanced chemical equation. first, we set all coefficients to 1: Zn + hcl = zncl2 + h2. balance the following equation:zn + hcl → zncl2 +. to balance the chemical equation zn + hcl = zncl2 + h2 you first. Zn (s) +. Balancing Equation Zn Hcl Zncl2 H2.
From www.numerade.com
SOLVED The equation below shows the reaction of zinc with hydrochloric Balancing Equation Zn Hcl Zncl2 H2 first, we set all coefficients to 1: Zn + hcl = zncl2 + h2. zinc metal reacts with hydrochloric acid according to the balanced equation: 1 zn + 1 hcl = 1 h 2 + 1 zncl 2. Solved and balanced chemical equation. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of. Balancing Equation Zn Hcl Zncl2 H2.
From www.chegg.com
Solved When you balance the equation Zn+HCl→ZnCl2+H2, what Balancing Equation Zn Hcl Zncl2 H2 Solved and balanced chemical equation. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. zinc metal reacts with hydrochloric acid according to the balanced equation: first, we set all coefficients to 1: balance the following equation:zn + hcl → zncl2 +. The balanced equation will be. Balancing Equation Zn Hcl Zncl2 H2.
From www.slideserve.com
PPT Balancing Redox Equations PowerPoint Presentation, free download Balancing Equation Zn Hcl Zncl2 H2 first, we set all coefficients to 1: 1 zn + 1 hcl = 1 h 2 + 1 zncl 2. The balanced equation will be calculated along. Zn + hcl = zncl2 + h2. Solved and balanced chemical equation. balance the following equation:zn + hcl → zncl2 +. to balance the chemical equation zn + hcl =. Balancing Equation Zn Hcl Zncl2 H2.