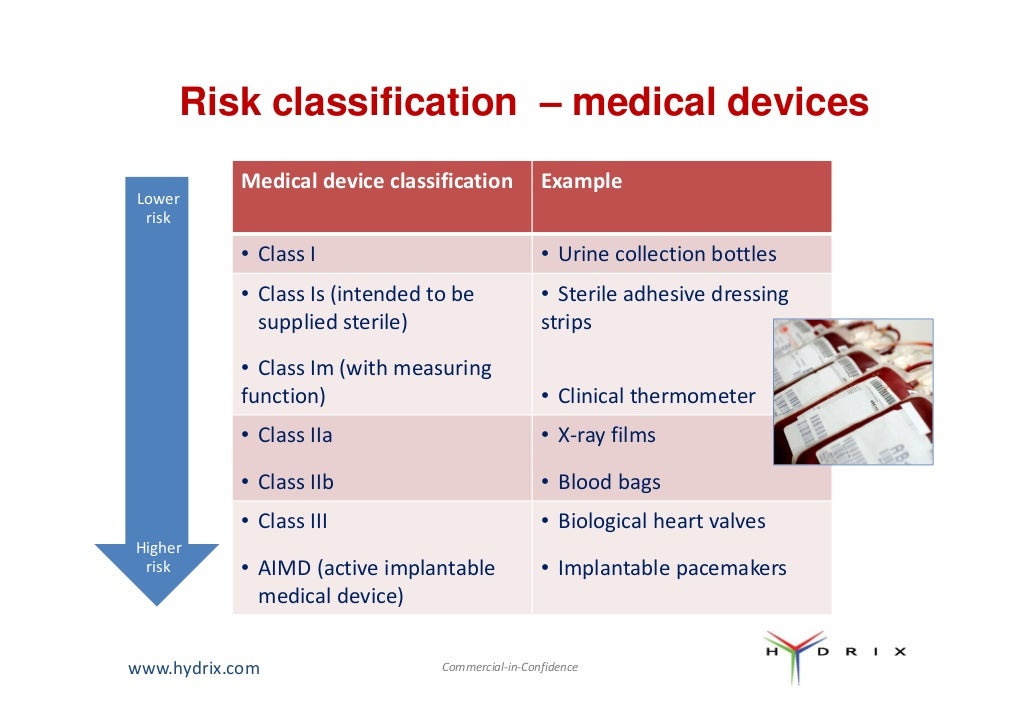
Medical Device Regulation List . In exercise of the powers conferred by sections 45, 71 and 72 of the health products. Listed below are some of the most recent publications supporting the implementation of the eu mdr. Here is the list of guidance documents with relevant forms and templates to help you meet the regulatory requirements for. Manufacturers must comply with the regulation when placing new medical devices. We regulate medical devices in singapore under the health products act (hpa) and its health products (medical devices) regulations 2010. Global atlas of medical devices book provides the global, regional and country data particularly on the availability of specific medical devices, policies, guidelines,. Health products (medical devices) regulations 2010. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical devices regulation applies since 26 may 2021. Harmonised standards under the regulations on medical devices and in vitro diagnostic medical devices are developed by.
from www.slideshare.net
Here is the list of guidance documents with relevant forms and templates to help you meet the regulatory requirements for. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical devices regulation applies since 26 may 2021. Manufacturers must comply with the regulation when placing new medical devices. We regulate medical devices in singapore under the health products act (hpa) and its health products (medical devices) regulations 2010. In exercise of the powers conferred by sections 45, 71 and 72 of the health products. Health products (medical devices) regulations 2010. Listed below are some of the most recent publications supporting the implementation of the eu mdr. Harmonised standards under the regulations on medical devices and in vitro diagnostic medical devices are developed by. Global atlas of medical devices book provides the global, regional and country data particularly on the availability of specific medical devices, policies, guidelines,.
Medical Device Regulation
Medical Device Regulation List Manufacturers must comply with the regulation when placing new medical devices. We regulate medical devices in singapore under the health products act (hpa) and its health products (medical devices) regulations 2010. Listed below are some of the most recent publications supporting the implementation of the eu mdr. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Manufacturers must comply with the regulation when placing new medical devices. Here is the list of guidance documents with relevant forms and templates to help you meet the regulatory requirements for. Harmonised standards under the regulations on medical devices and in vitro diagnostic medical devices are developed by. The medical devices regulation applies since 26 may 2021. In exercise of the powers conferred by sections 45, 71 and 72 of the health products. Health products (medical devices) regulations 2010. Global atlas of medical devices book provides the global, regional and country data particularly on the availability of specific medical devices, policies, guidelines,.
From www.lek.com
European Medical Devices Regulations and Their Impact Medical Device Regulation List Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In exercise of the powers conferred by sections 45, 71 and 72 of the health products. Global atlas of medical devices book provides the global, regional and country data particularly on the availability of specific medical devices, policies, guidelines,. Harmonised. Medical Device Regulation List.
From www.johner-institute.com
Medical Device Regulation MDR Medical Device Regulation List Global atlas of medical devices book provides the global, regional and country data particularly on the availability of specific medical devices, policies, guidelines,. Harmonised standards under the regulations on medical devices and in vitro diagnostic medical devices are developed by. Listed below are some of the most recent publications supporting the implementation of the eu mdr. Regulation (eu) 2017/745 of. Medical Device Regulation List.
From alirahealth.com
Medical Device Regulation (MDR) Support Alira Health Medical Device Regulation List The medical devices regulation applies since 26 may 2021. Listed below are some of the most recent publications supporting the implementation of the eu mdr. Harmonised standards under the regulations on medical devices and in vitro diagnostic medical devices are developed by. Health products (medical devices) regulations 2010. In exercise of the powers conferred by sections 45, 71 and 72. Medical Device Regulation List.
From kladuvsja.blob.core.windows.net
Medical Device Regulation Eu at Hay blog Medical Device Regulation List Manufacturers must comply with the regulation when placing new medical devices. In exercise of the powers conferred by sections 45, 71 and 72 of the health products. We regulate medical devices in singapore under the health products act (hpa) and its health products (medical devices) regulations 2010. Health products (medical devices) regulations 2010. The medical devices regulation applies since 26. Medical Device Regulation List.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification Medical Device Regulation List In exercise of the powers conferred by sections 45, 71 and 72 of the health products. Harmonised standards under the regulations on medical devices and in vitro diagnostic medical devices are developed by. The medical devices regulation applies since 26 may 2021. Here is the list of guidance documents with relevant forms and templates to help you meet the regulatory. Medical Device Regulation List.
From fr.slideshare.net
Medical Device FDA Regulations and Classifications infographic Medical Device Regulation List The medical devices regulation applies since 26 may 2021. We regulate medical devices in singapore under the health products act (hpa) and its health products (medical devices) regulations 2010. Harmonised standards under the regulations on medical devices and in vitro diagnostic medical devices are developed by. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april. Medical Device Regulation List.
From reviewmotors.co
Medical Device Directive Spare Parts List Reviewmotors.co Medical Device Regulation List Here is the list of guidance documents with relevant forms and templates to help you meet the regulatory requirements for. Harmonised standards under the regulations on medical devices and in vitro diagnostic medical devices are developed by. Manufacturers must comply with the regulation when placing new medical devices. We regulate medical devices in singapore under the health products act (hpa). Medical Device Regulation List.
From www.pacificbridgemedical.com
Device Classification in India Infographic Medical Device Regulation List Global atlas of medical devices book provides the global, regional and country data particularly on the availability of specific medical devices, policies, guidelines,. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical devices regulation applies since 26 may 2021. Manufacturers must comply with the regulation when placing. Medical Device Regulation List.
From es.slideshare.net
Regulation of Medical Devices in US Medical Device Regulation List The medical devices regulation applies since 26 may 2021. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Manufacturers must comply with the regulation when placing new medical devices. Global atlas of medical devices book provides the global, regional and country data particularly on the availability of specific medical. Medical Device Regulation List.
From www.arenasolutions.com
How to Classify Your Medical Device for FDA Approval Arena Medical Device Regulation List Harmonised standards under the regulations on medical devices and in vitro diagnostic medical devices are developed by. Health products (medical devices) regulations 2010. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Global atlas of medical devices book provides the global, regional and country data particularly on the availability. Medical Device Regulation List.
From www.slideserve.com
PPT Overview of FDA Device Regulations PowerPoint Presentation, free Medical Device Regulation List We regulate medical devices in singapore under the health products act (hpa) and its health products (medical devices) regulations 2010. Here is the list of guidance documents with relevant forms and templates to help you meet the regulatory requirements for. The medical devices regulation applies since 26 may 2021. Listed below are some of the most recent publications supporting the. Medical Device Regulation List.
From www.slideshare.net
Australia medical device approval chart Emergo Group Medical Device Regulation List Manufacturers must comply with the regulation when placing new medical devices. Here is the list of guidance documents with relevant forms and templates to help you meet the regulatory requirements for. In exercise of the powers conferred by sections 45, 71 and 72 of the health products. Health products (medical devices) regulations 2010. The medical devices regulation applies since 26. Medical Device Regulation List.
From www.bmedicalsystems.com
FAQ on the European Medical Device Regulation B Medical Systems (US) Medical Device Regulation List Manufacturers must comply with the regulation when placing new medical devices. Health products (medical devices) regulations 2010. Harmonised standards under the regulations on medical devices and in vitro diagnostic medical devices are developed by. The medical devices regulation applies since 26 may 2021. Here is the list of guidance documents with relevant forms and templates to help you meet the. Medical Device Regulation List.
From angelanjohnson.com
Medical Devices Angela N Johnson Medical Device Regulation List In exercise of the powers conferred by sections 45, 71 and 72 of the health products. We regulate medical devices in singapore under the health products act (hpa) and its health products (medical devices) regulations 2010. Harmonised standards under the regulations on medical devices and in vitro diagnostic medical devices are developed by. Regulation (eu) 2017/745 of the european parliament. Medical Device Regulation List.
From readmagazine.com
Unlocking the Secrets of Medical Device Regulations A Comprehensive Medical Device Regulation List Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Health products (medical devices) regulations 2010. The medical devices regulation applies since 26 may 2021. Here is the list of guidance documents with relevant forms and templates to help you meet the regulatory requirements for. Harmonised standards under the regulations. Medical Device Regulation List.
From readmagazine.com
Unlocking the Secrets of Medical Device Regulations A Comprehensive Medical Device Regulation List Harmonised standards under the regulations on medical devices and in vitro diagnostic medical devices are developed by. Here is the list of guidance documents with relevant forms and templates to help you meet the regulatory requirements for. Listed below are some of the most recent publications supporting the implementation of the eu mdr. Manufacturers must comply with the regulation when. Medical Device Regulation List.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Medical Device Regulation List In exercise of the powers conferred by sections 45, 71 and 72 of the health products. We regulate medical devices in singapore under the health products act (hpa) and its health products (medical devices) regulations 2010. Here is the list of guidance documents with relevant forms and templates to help you meet the regulatory requirements for. Manufacturers must comply with. Medical Device Regulation List.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Medical Device Regulation List In exercise of the powers conferred by sections 45, 71 and 72 of the health products. We regulate medical devices in singapore under the health products act (hpa) and its health products (medical devices) regulations 2010. Listed below are some of the most recent publications supporting the implementation of the eu mdr. Manufacturers must comply with the regulation when placing. Medical Device Regulation List.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Regulation List Harmonised standards under the regulations on medical devices and in vitro diagnostic medical devices are developed by. Here is the list of guidance documents with relevant forms and templates to help you meet the regulatory requirements for. We regulate medical devices in singapore under the health products act (hpa) and its health products (medical devices) regulations 2010. Regulation (eu) 2017/745. Medical Device Regulation List.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Medical Device Regulation List Listed below are some of the most recent publications supporting the implementation of the eu mdr. Here is the list of guidance documents with relevant forms and templates to help you meet the regulatory requirements for. Health products (medical devices) regulations 2010. In exercise of the powers conferred by sections 45, 71 and 72 of the health products. Regulation (eu). Medical Device Regulation List.
From www.youtube.com
Medical Devices classification as per FDA Medical Device Regulations Medical Device Regulation List Harmonised standards under the regulations on medical devices and in vitro diagnostic medical devices are developed by. Health products (medical devices) regulations 2010. Manufacturers must comply with the regulation when placing new medical devices. We regulate medical devices in singapore under the health products act (hpa) and its health products (medical devices) regulations 2010. Listed below are some of the. Medical Device Regulation List.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Regulation List We regulate medical devices in singapore under the health products act (hpa) and its health products (medical devices) regulations 2010. Harmonised standards under the regulations on medical devices and in vitro diagnostic medical devices are developed by. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Listed below are. Medical Device Regulation List.
From www.slideserve.com
PPT Medical Device Standards PowerPoint Presentation, free download Medical Device Regulation List Global atlas of medical devices book provides the global, regional and country data particularly on the availability of specific medical devices, policies, guidelines,. The medical devices regulation applies since 26 may 2021. Harmonised standards under the regulations on medical devices and in vitro diagnostic medical devices are developed by. We regulate medical devices in singapore under the health products act. Medical Device Regulation List.
From globalpccs.com
EU Medical Device Regulation Compliance Services in IMDS CDX ELV Medical Device Regulation List Listed below are some of the most recent publications supporting the implementation of the eu mdr. We regulate medical devices in singapore under the health products act (hpa) and its health products (medical devices) regulations 2010. Manufacturers must comply with the regulation when placing new medical devices. Regulation (eu) 2017/745 of the european parliament and of the council of 5. Medical Device Regulation List.
From joiezthhz.blob.core.windows.net
Standard Medical Device Regulation at Moises Cox blog Medical Device Regulation List Listed below are some of the most recent publications supporting the implementation of the eu mdr. Manufacturers must comply with the regulation when placing new medical devices. The medical devices regulation applies since 26 may 2021. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Global atlas of medical. Medical Device Regulation List.
From betebt.com
Medical Device Regulation Importance and Examples in APAC (2022) Medical Device Regulation List The medical devices regulation applies since 26 may 2021. Health products (medical devices) regulations 2010. In exercise of the powers conferred by sections 45, 71 and 72 of the health products. Global atlas of medical devices book provides the global, regional and country data particularly on the availability of specific medical devices, policies, guidelines,. Manufacturers must comply with the regulation. Medical Device Regulation List.
From crfweb.com
Medical Device Regulations Medical Device Regulation List Harmonised standards under the regulations on medical devices and in vitro diagnostic medical devices are developed by. The medical devices regulation applies since 26 may 2021. Global atlas of medical devices book provides the global, regional and country data particularly on the availability of specific medical devices, policies, guidelines,. Regulation (eu) 2017/745 of the european parliament and of the council. Medical Device Regulation List.
From www.researchsolutions.com
European Medical Device Regulation Guide to simplify compliance 2021 Medical Device Regulation List Here is the list of guidance documents with relevant forms and templates to help you meet the regulatory requirements for. In exercise of the powers conferred by sections 45, 71 and 72 of the health products. Listed below are some of the most recent publications supporting the implementation of the eu mdr. Global atlas of medical devices book provides the. Medical Device Regulation List.
From www.slideshare.net
Medical Device Regulation Medical Device Regulation List In exercise of the powers conferred by sections 45, 71 and 72 of the health products. We regulate medical devices in singapore under the health products act (hpa) and its health products (medical devices) regulations 2010. The medical devices regulation applies since 26 may 2021. Global atlas of medical devices book provides the global, regional and country data particularly on. Medical Device Regulation List.
From www.qualio.com
The 3 FDA medical device classes differences and examples explained Medical Device Regulation List In exercise of the powers conferred by sections 45, 71 and 72 of the health products. Manufacturers must comply with the regulation when placing new medical devices. Listed below are some of the most recent publications supporting the implementation of the eu mdr. Harmonised standards under the regulations on medical devices and in vitro diagnostic medical devices are developed by.. Medical Device Regulation List.
From www.cell.com
The Regulation of Wearable Medical Devices Trends in Biotechnology Medical Device Regulation List Harmonised standards under the regulations on medical devices and in vitro diagnostic medical devices are developed by. Health products (medical devices) regulations 2010. We regulate medical devices in singapore under the health products act (hpa) and its health products (medical devices) regulations 2010. Global atlas of medical devices book provides the global, regional and country data particularly on the availability. Medical Device Regulation List.
From medicaldevicehq.com
Medical Device Regulation codes Medical Device HQ 1 Medical Device Regulation List Listed below are some of the most recent publications supporting the implementation of the eu mdr. The medical devices regulation applies since 26 may 2021. Global atlas of medical devices book provides the global, regional and country data particularly on the availability of specific medical devices, policies, guidelines,. In exercise of the powers conferred by sections 45, 71 and 72. Medical Device Regulation List.
From www.meddeviceonline.com
FDA's Safety And Performancebased Pathway An Alternative To Medical Device Regulation List Harmonised standards under the regulations on medical devices and in vitro diagnostic medical devices are developed by. The medical devices regulation applies since 26 may 2021. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Listed below are some of the most recent publications supporting the implementation of the. Medical Device Regulation List.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Regulation List The medical devices regulation applies since 26 may 2021. Global atlas of medical devices book provides the global, regional and country data particularly on the availability of specific medical devices, policies, guidelines,. Health products (medical devices) regulations 2010. Listed below are some of the most recent publications supporting the implementation of the eu mdr. Here is the list of guidance. Medical Device Regulation List.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Regulation List In exercise of the powers conferred by sections 45, 71 and 72 of the health products. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Listed below are some of the most recent publications supporting the implementation of the eu mdr. Here is the list of guidance documents with. Medical Device Regulation List.