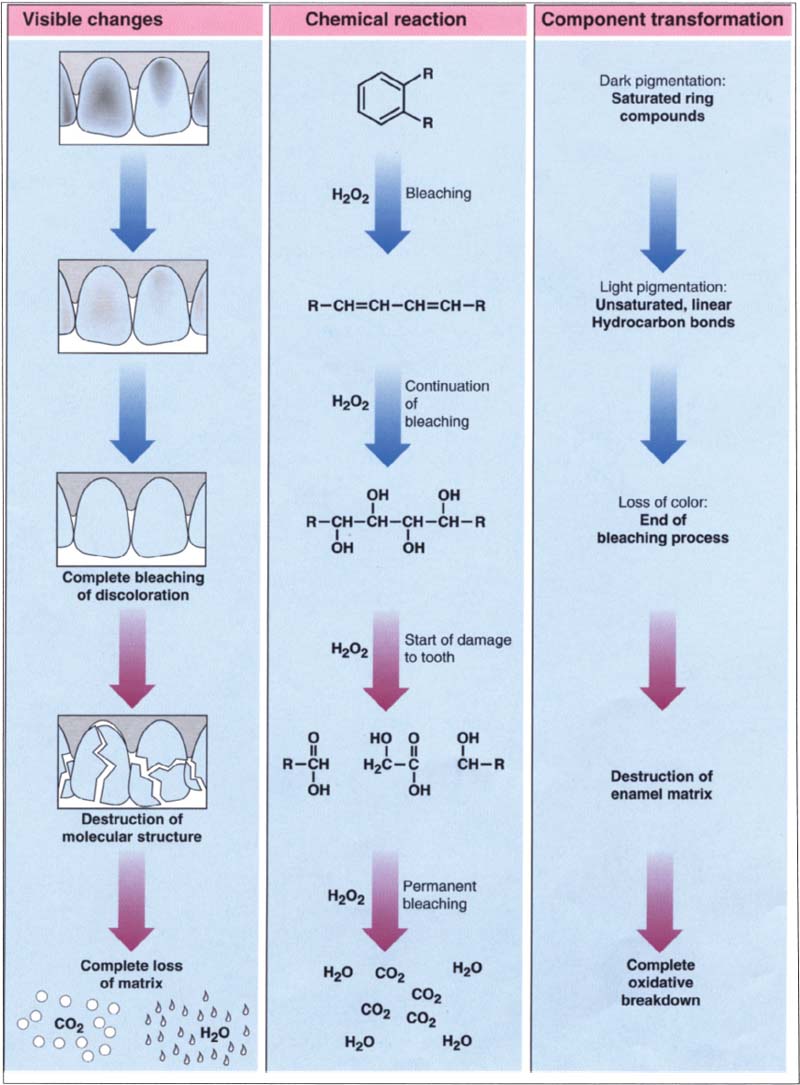
Bleaching On Chemistry . Bleaching agents are commonly used in the paper and pulp industry, the textile industry, and the commercial and household laundering. Bleaching agents essentially destroy chromophores (thereby removing the color), via the oxidation or reduction of these absorbing groups. Put simply, the bleaching process disintegrates the molecular bonds of chromophores. This happens as a result of either an oxidation reaction or a reduction reaction. Thus, bleaches can be classified as either. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the formation of dioxin waste. Bleach, solid or liquid chemical used to whiten or remove the natural color of fibers, yarns, paper, and textile fabrics or to remove.
from pocketdentistry.com
Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the formation of dioxin waste. Bleaching agents are commonly used in the paper and pulp industry, the textile industry, and the commercial and household laundering. Put simply, the bleaching process disintegrates the molecular bonds of chromophores. This happens as a result of either an oxidation reaction or a reduction reaction. Bleaching agents essentially destroy chromophores (thereby removing the color), via the oxidation or reduction of these absorbing groups. Bleach, solid or liquid chemical used to whiten or remove the natural color of fibers, yarns, paper, and textile fabrics or to remove. Thus, bleaches can be classified as either.
Bleaching Pocket Dentistry
Bleaching On Chemistry Bleach, solid or liquid chemical used to whiten or remove the natural color of fibers, yarns, paper, and textile fabrics or to remove. Bleach, solid or liquid chemical used to whiten or remove the natural color of fibers, yarns, paper, and textile fabrics or to remove. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the formation of dioxin waste. Thus, bleaches can be classified as either. Bleaching agents essentially destroy chromophores (thereby removing the color), via the oxidation or reduction of these absorbing groups. Bleaching agents are commonly used in the paper and pulp industry, the textile industry, and the commercial and household laundering. Put simply, the bleaching process disintegrates the molecular bonds of chromophores. This happens as a result of either an oxidation reaction or a reduction reaction.
From www.youtube.com
Bleaching Powder Class 10 Acid Base and Salt Chemistry YouTube Bleaching On Chemistry Bleaching agents essentially destroy chromophores (thereby removing the color), via the oxidation or reduction of these absorbing groups. Bleach, solid or liquid chemical used to whiten or remove the natural color of fibers, yarns, paper, and textile fabrics or to remove. Thus, bleaches can be classified as either. This happens as a result of either an oxidation reaction or a. Bleaching On Chemistry.
From www.youtube.com
Bleaching Powder Hasenclever's Method Chapter 5 Chemistry Class Bleaching On Chemistry Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the formation of dioxin waste. Put simply, the bleaching process disintegrates the molecular bonds of chromophores. Bleaching agents are commonly used in the paper and pulp industry, the textile industry, and the commercial and household laundering. Thus, bleaches can be classified. Bleaching On Chemistry.
From www.studocu.com
Chemistry project sterilization of water using bleaching powder pdf Bleaching On Chemistry Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the formation of dioxin waste. This happens as a result of either an oxidation reaction or a reduction reaction. Bleaching agents are commonly used in the paper and pulp industry, the textile industry, and the commercial and household laundering. Bleaching agents. Bleaching On Chemistry.
From www.youtube.com
Physical Properties of Bleaching Powder, Chemistry Lecture Sabaq.pk Bleaching On Chemistry Bleaching agents are commonly used in the paper and pulp industry, the textile industry, and the commercial and household laundering. Thus, bleaches can be classified as either. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the formation of dioxin waste. This happens as a result of either an oxidation. Bleaching On Chemistry.
From www.slideserve.com
PPT Chemical pulp bleaching PowerPoint Presentation, free download Bleaching On Chemistry Put simply, the bleaching process disintegrates the molecular bonds of chromophores. Bleaching agents are commonly used in the paper and pulp industry, the textile industry, and the commercial and household laundering. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the formation of dioxin waste. This happens as a result. Bleaching On Chemistry.
From www.slideserve.com
PPT Chemical pulp bleaching PowerPoint Presentation, free download Bleaching On Chemistry Put simply, the bleaching process disintegrates the molecular bonds of chromophores. Bleach, solid or liquid chemical used to whiten or remove the natural color of fibers, yarns, paper, and textile fabrics or to remove. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the formation of dioxin waste. Bleaching agents. Bleaching On Chemistry.
From www.masterorganicchemistry.com
Conjugation And Color (+ How Bleach Works) Master Organic Chemistry Bleaching On Chemistry Thus, bleaches can be classified as either. Bleaching agents are commonly used in the paper and pulp industry, the textile industry, and the commercial and household laundering. Bleaching agents essentially destroy chromophores (thereby removing the color), via the oxidation or reduction of these absorbing groups. This happens as a result of either an oxidation reaction or a reduction reaction. Bleaching. Bleaching On Chemistry.
From www.youtube.com
Q. Explain the bleaching action of SO2 ? YouTube Bleaching On Chemistry Thus, bleaches can be classified as either. Bleaching agents are commonly used in the paper and pulp industry, the textile industry, and the commercial and household laundering. This happens as a result of either an oxidation reaction or a reduction reaction. Bleach, solid or liquid chemical used to whiten or remove the natural color of fibers, yarns, paper, and textile. Bleaching On Chemistry.
From www.slideserve.com
PPT Pulping and Bleaching PSE 476/Chem E 471 PowerPoint Presentation Bleaching On Chemistry Bleaching agents are commonly used in the paper and pulp industry, the textile industry, and the commercial and household laundering. Bleaching agents essentially destroy chromophores (thereby removing the color), via the oxidation or reduction of these absorbing groups. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the formation of. Bleaching On Chemistry.
From www.youtube.com
Making Bleach AS Chemistry YouTube Bleaching On Chemistry Thus, bleaches can be classified as either. This happens as a result of either an oxidation reaction or a reduction reaction. Bleach, solid or liquid chemical used to whiten or remove the natural color of fibers, yarns, paper, and textile fabrics or to remove. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process,. Bleaching On Chemistry.
From www.slideserve.com
PPT Pulping and Bleaching PSE 476/Chem E 471 PowerPoint Presentation Bleaching On Chemistry Put simply, the bleaching process disintegrates the molecular bonds of chromophores. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the formation of dioxin waste. Thus, bleaches can be classified as either. Bleach, solid or liquid chemical used to whiten or remove the natural color of fibers, yarns, paper, and. Bleaching On Chemistry.
From www.youtube.com
Uses of Bleaching Powder Grade 12 Chemistry Lecture 30 YouTube Bleaching On Chemistry Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the formation of dioxin waste. This happens as a result of either an oxidation reaction or a reduction reaction. Put simply, the bleaching process disintegrates the molecular bonds of chromophores. Thus, bleaches can be classified as either. Bleaching agents are commonly. Bleaching On Chemistry.
From www.youtube.com
sterilization of water using bleaching powder chemistry investigatory Bleaching On Chemistry This happens as a result of either an oxidation reaction or a reduction reaction. Bleach, solid or liquid chemical used to whiten or remove the natural color of fibers, yarns, paper, and textile fabrics or to remove. Thus, bleaches can be classified as either. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process,. Bleaching On Chemistry.
From www.slideserve.com
PPT Chemical pulp bleaching PowerPoint Presentation, free download Bleaching On Chemistry Bleach, solid or liquid chemical used to whiten or remove the natural color of fibers, yarns, paper, and textile fabrics or to remove. This happens as a result of either an oxidation reaction or a reduction reaction. Bleaching agents are commonly used in the paper and pulp industry, the textile industry, and the commercial and household laundering. Bleaching agents essentially. Bleaching On Chemistry.
From www.slideserve.com
PPT Mechanical Pulping and Pulp Bleaching Chemistry PowerPoint Bleaching On Chemistry Bleaching agents are commonly used in the paper and pulp industry, the textile industry, and the commercial and household laundering. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the formation of dioxin waste. This happens as a result of either an oxidation reaction or a reduction reaction. Put simply,. Bleaching On Chemistry.
From www.slideserve.com
PPT Chemical pulp bleaching PowerPoint Presentation, free download Bleaching On Chemistry Put simply, the bleaching process disintegrates the molecular bonds of chromophores. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the formation of dioxin waste. Bleaching agents are commonly used in the paper and pulp industry, the textile industry, and the commercial and household laundering. Bleaching agents essentially destroy chromophores. Bleaching On Chemistry.
From www.youtube.com
12th Class Chemistry Chapter 5 BLEACHING POWDER 2nd Year Chemistry Bleaching On Chemistry This happens as a result of either an oxidation reaction or a reduction reaction. Bleaching agents essentially destroy chromophores (thereby removing the color), via the oxidation or reduction of these absorbing groups. Put simply, the bleaching process disintegrates the molecular bonds of chromophores. Bleaching agents are commonly used in the paper and pulp industry, the textile industry, and the commercial. Bleaching On Chemistry.
From www.youtube.com
Reaction of Bleaching Powder with Carbondioxide and Water, Chemistry Bleaching On Chemistry This happens as a result of either an oxidation reaction or a reduction reaction. Bleach, solid or liquid chemical used to whiten or remove the natural color of fibers, yarns, paper, and textile fabrics or to remove. Thus, bleaches can be classified as either. Put simply, the bleaching process disintegrates the molecular bonds of chromophores. Bleaching agents essentially destroy chromophores. Bleaching On Chemistry.
From www.scribd.com
Class 12th Chemistry Project On Sterilization of Water by Using Bleaching On Chemistry Bleaching agents are commonly used in the paper and pulp industry, the textile industry, and the commercial and household laundering. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the formation of dioxin waste. Put simply, the bleaching process disintegrates the molecular bonds of chromophores. Bleaching agents essentially destroy chromophores. Bleaching On Chemistry.
From www.youtube.com
Bleaching Powder Preparation and Mechanism Basic Chemistry YouTube Bleaching On Chemistry Bleach, solid or liquid chemical used to whiten or remove the natural color of fibers, yarns, paper, and textile fabrics or to remove. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the formation of dioxin waste. Put simply, the bleaching process disintegrates the molecular bonds of chromophores. Bleaching agents. Bleaching On Chemistry.
From byjus.com
5 What is the product of reaction of ethanol with bleaching powder? Bleaching On Chemistry Thus, bleaches can be classified as either. Bleaching agents are commonly used in the paper and pulp industry, the textile industry, and the commercial and household laundering. Put simply, the bleaching process disintegrates the molecular bonds of chromophores. This happens as a result of either an oxidation reaction or a reduction reaction. Bleach, solid or liquid chemical used to whiten. Bleaching On Chemistry.
From www.youtube.com
Sterilization of water using bleaching powder. ppt 2 YouTube Bleaching On Chemistry Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the formation of dioxin waste. Bleaching agents are commonly used in the paper and pulp industry, the textile industry, and the commercial and household laundering. Put simply, the bleaching process disintegrates the molecular bonds of chromophores. Thus, bleaches can be classified. Bleaching On Chemistry.
From www.slideserve.com
PPT Pulping and Bleaching PSE 476 PowerPoint Presentation, free Bleaching On Chemistry Bleaching agents essentially destroy chromophores (thereby removing the color), via the oxidation or reduction of these absorbing groups. Bleach, solid or liquid chemical used to whiten or remove the natural color of fibers, yarns, paper, and textile fabrics or to remove. Thus, bleaches can be classified as either. Bleaching agents are commonly used in the paper and pulp industry, the. Bleaching On Chemistry.
From www.youtube.com
Bleach and Hydrogen Peroxide Reaction + Balanced Equation YouTube Bleaching On Chemistry Bleaching agents are commonly used in the paper and pulp industry, the textile industry, and the commercial and household laundering. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the formation of dioxin waste. Bleach, solid or liquid chemical used to whiten or remove the natural color of fibers, yarns,. Bleaching On Chemistry.
From in.pinterest.com
Bleaching Powder Bleaching powder, Chemical structure, Molar mass Bleaching On Chemistry Put simply, the bleaching process disintegrates the molecular bonds of chromophores. Bleach, solid or liquid chemical used to whiten or remove the natural color of fibers, yarns, paper, and textile fabrics or to remove. Bleaching agents essentially destroy chromophores (thereby removing the color), via the oxidation or reduction of these absorbing groups. Thus, bleaches can be classified as either. Bleaching. Bleaching On Chemistry.
From sciencenotes.org
Oxygen Bleach vs Chlorine Bleach Bleaching On Chemistry This happens as a result of either an oxidation reaction or a reduction reaction. Bleaching agents are commonly used in the paper and pulp industry, the textile industry, and the commercial and household laundering. Thus, bleaches can be classified as either. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating. Bleaching On Chemistry.
From www.scribd.com
STERILIZATION OF WATER USING BLEACHING POWDER Chemistry Project PDF Bleaching On Chemistry Bleaching agents are commonly used in the paper and pulp industry, the textile industry, and the commercial and household laundering. This happens as a result of either an oxidation reaction or a reduction reaction. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the formation of dioxin waste. Bleach, solid. Bleaching On Chemistry.
From www.youtube.com
Reaction of Bleaching Powder with Water, Chemistry Lecture Sabaq.pk Bleaching On Chemistry This happens as a result of either an oxidation reaction or a reduction reaction. Bleaching agents are commonly used in the paper and pulp industry, the textile industry, and the commercial and household laundering. Put simply, the bleaching process disintegrates the molecular bonds of chromophores. Bleach, solid or liquid chemical used to whiten or remove the natural color of fibers,. Bleaching On Chemistry.
From www.slideserve.com
PPT Mechanical Pulping and Pulp Bleaching Chemistry PowerPoint Bleaching On Chemistry Bleach, solid or liquid chemical used to whiten or remove the natural color of fibers, yarns, paper, and textile fabrics or to remove. This happens as a result of either an oxidation reaction or a reduction reaction. Put simply, the bleaching process disintegrates the molecular bonds of chromophores. Thus, bleaches can be classified as either. Bleaching agents essentially destroy chromophores. Bleaching On Chemistry.
From www.slideserve.com
PPT Pulping and Bleaching PSE 476 PowerPoint Presentation, free Bleaching On Chemistry Bleach, solid or liquid chemical used to whiten or remove the natural color of fibers, yarns, paper, and textile fabrics or to remove. This happens as a result of either an oxidation reaction or a reduction reaction. Thus, bleaches can be classified as either. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process,. Bleaching On Chemistry.
From pocketdentistry.com
Bleaching Pocket Dentistry Bleaching On Chemistry Put simply, the bleaching process disintegrates the molecular bonds of chromophores. Bleaching agents are commonly used in the paper and pulp industry, the textile industry, and the commercial and household laundering. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the formation of dioxin waste. Bleaching agents essentially destroy chromophores. Bleaching On Chemistry.
From www.youtube.com
Bleaching powder Class 10 Ch. 2 Chemistry NCERT YouTube Bleaching On Chemistry Thus, bleaches can be classified as either. Bleaching agents essentially destroy chromophores (thereby removing the color), via the oxidation or reduction of these absorbing groups. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the formation of dioxin waste. Bleaching agents are commonly used in the paper and pulp industry,. Bleaching On Chemistry.
From www.slideserve.com
PPT Mechanical Pulping and Pulp Bleaching Chemistry PowerPoint Bleaching On Chemistry Put simply, the bleaching process disintegrates the molecular bonds of chromophores. Bleach, solid or liquid chemical used to whiten or remove the natural color of fibers, yarns, paper, and textile fabrics or to remove. Bleaching agents essentially destroy chromophores (thereby removing the color), via the oxidation or reduction of these absorbing groups. Bleaching agents are commonly used in the paper. Bleaching On Chemistry.
From www.academia.edu
(DOC) STERILIZATION OF WATER USING BLEACHING POWDER A CHEMISTRY Bleaching On Chemistry Bleaching agents essentially destroy chromophores (thereby removing the color), via the oxidation or reduction of these absorbing groups. This happens as a result of either an oxidation reaction or a reduction reaction. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the formation of dioxin waste. Put simply, the bleaching. Bleaching On Chemistry.
From www.youtube.com
Determination of percentage of Available chlorine in a sample of Bleaching On Chemistry Thus, bleaches can be classified as either. Bleaching agents essentially destroy chromophores (thereby removing the color), via the oxidation or reduction of these absorbing groups. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the formation of dioxin waste. Bleach, solid or liquid chemical used to whiten or remove the. Bleaching On Chemistry.