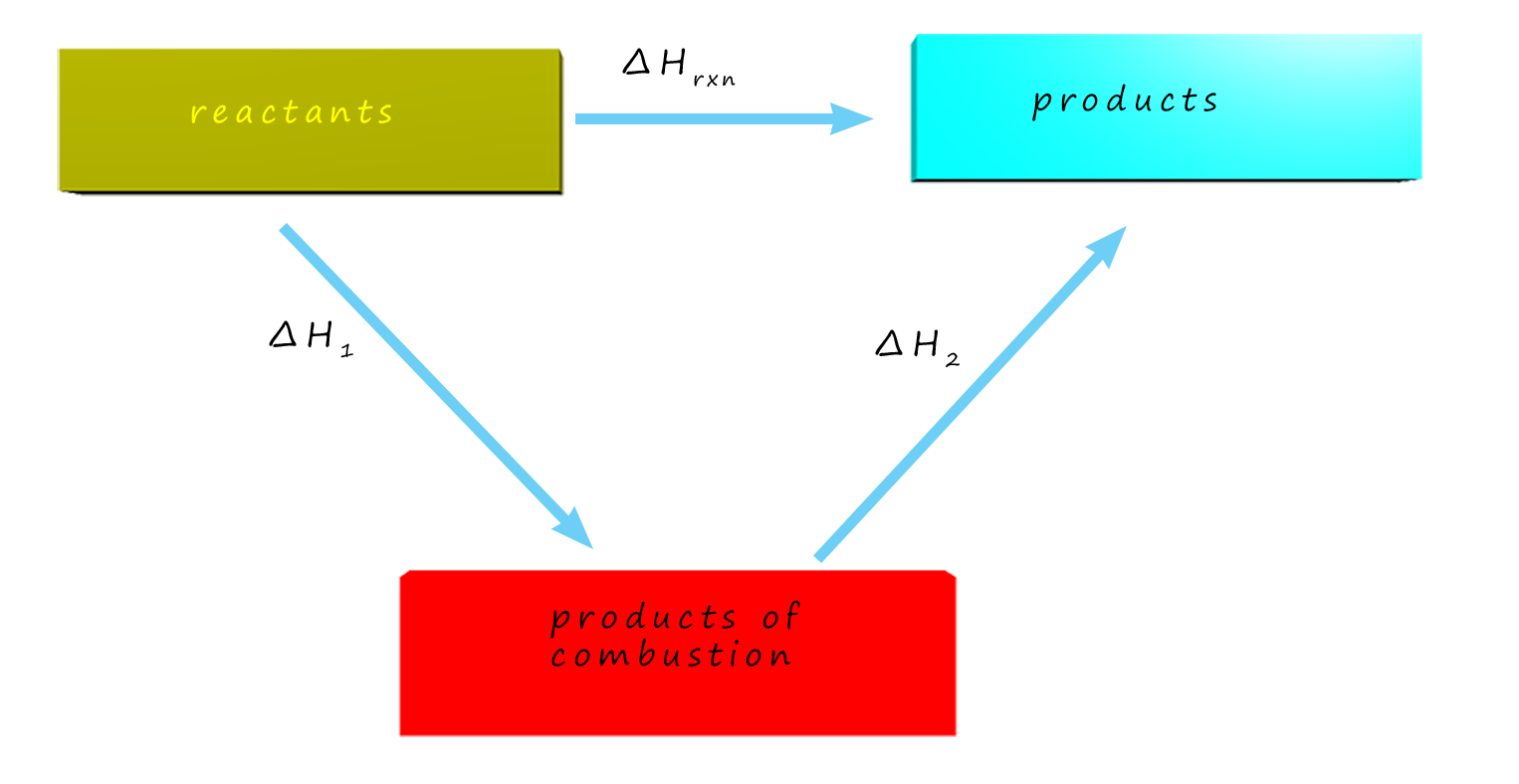
Enthalpy Of Combustion Hess Law . Explain hess’s law and use it to compute reaction enthalpies standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: For benzene, carbon and hydrogen, these are: This is one version of the first law of thermodynamics, and it shows that the internal energy of a system changes through heat flow. The key to solving this problem is to. Hess’s law tells us the overall enthalpy change for both routes will be the same. First you have to design your cycle. According to hess’s law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of the steps. We can apply the data from the experimental enthalpies of combustion in. According to hess’s law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of the steps. What is the enthalpy of combustion per mole of methane under these conditions? Write down the enthalpy change. Standard enthalpy changes of combustion, δh° c are relatively easy to measure. We can use enthalpies of combustion data to find the enthalpy changes for combustion reactions in the cycle. How much heat is produced when 100 ml of 0.250 m.
from www.science-revision.co.uk
What is the enthalpy of combustion per mole of methane under these conditions? How much heat is produced when 100 ml of 0.250 m. We can use enthalpies of combustion data to find the enthalpy changes for combustion reactions in the cycle. According to hess’s law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of the steps. The key to solving this problem is to. For benzene, carbon and hydrogen, these are: This is one version of the first law of thermodynamics, and it shows that the internal energy of a system changes through heat flow. First you have to design your cycle. According to hess’s law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of the steps. Explain hess’s law and use it to compute reaction enthalpies standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1.
Hess's Law
Enthalpy Of Combustion Hess Law It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: Write down the enthalpy change. This is one version of the first law of thermodynamics, and it shows that the internal energy of a system changes through heat flow. For benzene, carbon and hydrogen, these are: The key to solving this problem is to. What is the enthalpy of combustion per mole of methane under these conditions? Standard enthalpy changes of combustion, δh° c are relatively easy to measure. How much heat is produced when 100 ml of 0.250 m. We can apply the data from the experimental enthalpies of combustion in. First you have to design your cycle. Explain hess’s law and use it to compute reaction enthalpies standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1. Hess’s law tells us the overall enthalpy change for both routes will be the same. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: We can use enthalpies of combustion data to find the enthalpy changes for combustion reactions in the cycle. According to hess’s law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of the steps. According to hess’s law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of the steps.
From www.youtube.com
CHEMISTRY 101 Enthalpy of Reaction, Hess's Law YouTube Enthalpy Of Combustion Hess Law Write down the enthalpy change. Hess’s law tells us the overall enthalpy change for both routes will be the same. The key to solving this problem is to. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: Standard enthalpy changes of combustion, δh° c are relatively easy to measure. According. Enthalpy Of Combustion Hess Law.
From www.slideserve.com
PPT Hess ’ s Law Energetics PowerPoint Presentation, free download Enthalpy Of Combustion Hess Law Standard enthalpy changes of combustion, δh° c are relatively easy to measure. The key to solving this problem is to. According to hess’s law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of the steps. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol:. Enthalpy Of Combustion Hess Law.
From www.slideserve.com
PPT Hess’ Law of Heat Summation PowerPoint Presentation, free Enthalpy Of Combustion Hess Law For benzene, carbon and hydrogen, these are: What is the enthalpy of combustion per mole of methane under these conditions? First you have to design your cycle. Write down the enthalpy change. We can apply the data from the experimental enthalpies of combustion in. According to hess’s law, the enthalpy change of the reaction will equal the sum of the. Enthalpy Of Combustion Hess Law.
From www.numerade.com
SOLVED Find the enthalpy of combustion of magnesium using Hess' law Enthalpy Of Combustion Hess Law It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: Write down the enthalpy change. First you have to design your cycle. We can apply the data from the experimental enthalpies of combustion in. Hess’s law tells us the overall enthalpy change for both routes will be the same. Standard enthalpy. Enthalpy Of Combustion Hess Law.
From www.youtube.com
Hess's Law Problems & Enthalpy Change Chemistry YouTube Enthalpy Of Combustion Hess Law Standard enthalpy changes of combustion, δh° c are relatively easy to measure. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: For benzene, carbon and hydrogen, these are: This is one version of the first law of thermodynamics, and it shows that the internal energy of a system changes through. Enthalpy Of Combustion Hess Law.
From www.youtube.com
16.5 Hess' Law of Heat Summation Equation & Heat of Combustion YouTube Enthalpy Of Combustion Hess Law According to hess’s law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of the steps. How much heat is produced when 100 ml of 0.250 m. We can apply the data from the experimental enthalpies of combustion in. This is one version of the first law of thermodynamics, and it shows that the internal. Enthalpy Of Combustion Hess Law.
From www.slideserve.com
PPT Hess’s Law PowerPoint Presentation ID456765 Enthalpy Of Combustion Hess Law We can apply the data from the experimental enthalpies of combustion in. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: Standard enthalpy changes of combustion, δh° c are relatively easy to measure. Write down the enthalpy change. What is the enthalpy of combustion per mole of methane under these. Enthalpy Of Combustion Hess Law.
From www.youtube.com
killer stratergies Hess's law for calculating enthalpy of combustion Enthalpy Of Combustion Hess Law What is the enthalpy of combustion per mole of methane under these conditions? First you have to design your cycle. Explain hess’s law and use it to compute reaction enthalpies standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1. Write down the enthalpy change. We can apply the data from the experimental enthalpies of combustion in. According. Enthalpy Of Combustion Hess Law.
From www.youtube.com
Calculating Enthalpy of Combustion using Hess's Law YouTube Enthalpy Of Combustion Hess Law Write down the enthalpy change. Explain hess’s law and use it to compute reaction enthalpies standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1. This is one version of the first law of thermodynamics, and it shows that the internal energy of a system changes through heat flow. We can apply the data from the experimental enthalpies. Enthalpy Of Combustion Hess Law.
From www.youtube.com
Hess's law triangle problem YouTube Enthalpy Of Combustion Hess Law According to hess’s law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of the steps. We can apply the data from the experimental enthalpies of combustion in. Explain hess’s law and use it to compute reaction enthalpies standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1. For benzene, carbon and hydrogen,. Enthalpy Of Combustion Hess Law.
From www.slideserve.com
PPT Thermochemisty (Enthalpy) and Hess’s Law PowerPoint Presentation Enthalpy Of Combustion Hess Law We can use enthalpies of combustion data to find the enthalpy changes for combustion reactions in the cycle. The key to solving this problem is to. What is the enthalpy of combustion per mole of methane under these conditions? How much heat is produced when 100 ml of 0.250 m. Hess’s law tells us the overall enthalpy change for both. Enthalpy Of Combustion Hess Law.
From www.youtube.com
Hess's Law using enthalpies of combustion YouTube Enthalpy Of Combustion Hess Law First you have to design your cycle. Write down the enthalpy change. Hess’s law tells us the overall enthalpy change for both routes will be the same. Explain hess’s law and use it to compute reaction enthalpies standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1. It is so common that the phrase standard enthalpy of combustion. Enthalpy Of Combustion Hess Law.
From chemistryguru.com.sg
How to Determine Enthalpy Change of Reaction Using Hess' Law Enthalpy Of Combustion Hess Law Standard enthalpy changes of combustion, δh° c are relatively easy to measure. First you have to design your cycle. Write down the enthalpy change. What is the enthalpy of combustion per mole of methane under these conditions? For benzene, carbon and hydrogen, these are: How much heat is produced when 100 ml of 0.250 m. This is one version of. Enthalpy Of Combustion Hess Law.
From www.youtube.com
How to construct a Hess' Law Energy Cycle YouTube Enthalpy Of Combustion Hess Law Write down the enthalpy change. Explain hess’s law and use it to compute reaction enthalpies standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1. What is the enthalpy of combustion per mole of methane under these conditions? For benzene, carbon and hydrogen, these are: The key to solving this problem is to. This is one version of. Enthalpy Of Combustion Hess Law.
From www.chegg.com
Solved 5) Using Hess' Law, calculate the change in enthalpy Enthalpy Of Combustion Hess Law According to hess’s law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of the steps. First you have to design your cycle. For benzene, carbon and hydrogen, these are: According to hess’s law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of the steps. This is one version. Enthalpy Of Combustion Hess Law.
From chemistry.stackexchange.com
physical chemistry Why is the enthalpy of a reaction equal to the Enthalpy Of Combustion Hess Law According to hess’s law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of the steps. This is one version of the first law of thermodynamics, and it shows that the internal energy of a system changes through heat flow. First you have to design your cycle. Hess’s law tells us the overall enthalpy change. Enthalpy Of Combustion Hess Law.
From chem.libretexts.org
5.6 Hess's Law Chemistry LibreTexts Enthalpy Of Combustion Hess Law This is one version of the first law of thermodynamics, and it shows that the internal energy of a system changes through heat flow. We can use enthalpies of combustion data to find the enthalpy changes for combustion reactions in the cycle. What is the enthalpy of combustion per mole of methane under these conditions? How much heat is produced. Enthalpy Of Combustion Hess Law.
From slidetodoc.com
Thermochemistry 1 Hesss Law Heat of Formation Heat Enthalpy Of Combustion Hess Law We can use enthalpies of combustion data to find the enthalpy changes for combustion reactions in the cycle. According to hess’s law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of the steps. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: How much. Enthalpy Of Combustion Hess Law.
From www.chemistrystudent.com
Hess' Law and Hess Cycles (ALevel) ChemistryStudent Enthalpy Of Combustion Hess Law According to hess’s law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of the steps. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: First you have to design your cycle. This is one version of the first law of thermodynamics, and it shows. Enthalpy Of Combustion Hess Law.
From www.youtube.com
Hess's Law Calculations (Enthalpy of Combustion) YouTube Enthalpy Of Combustion Hess Law This is one version of the first law of thermodynamics, and it shows that the internal energy of a system changes through heat flow. First you have to design your cycle. Hess’s law tells us the overall enthalpy change for both routes will be the same. How much heat is produced when 100 ml of 0.250 m. According to hess’s. Enthalpy Of Combustion Hess Law.
From www.slideserve.com
PPT Lecture 4 Hess’ Law PowerPoint Presentation, free download ID Enthalpy Of Combustion Hess Law According to hess’s law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of the steps. First you have to design your cycle. According to hess’s law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of the steps. Write down the enthalpy change. Hess’s law tells us the overall. Enthalpy Of Combustion Hess Law.
From www.numerade.com
SOLVED Hess' Law questions Q1. Using the data in the table below Enthalpy Of Combustion Hess Law First you have to design your cycle. We can apply the data from the experimental enthalpies of combustion in. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: For benzene, carbon and hydrogen, these are: Write down the enthalpy change. We can use enthalpies of combustion data to find the. Enthalpy Of Combustion Hess Law.
From pressbooks.online.ucf.edu
10.4 Hess’s Law Chemistry Fundamentals Enthalpy Of Combustion Hess Law What is the enthalpy of combustion per mole of methane under these conditions? According to hess’s law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of the steps. How much heat is produced when 100 ml of 0.250 m. We can use enthalpies of combustion data to find the enthalpy changes for combustion reactions. Enthalpy Of Combustion Hess Law.
From www.sliderbase.com
Hess's Law Presentation Chemistry Enthalpy Of Combustion Hess Law How much heat is produced when 100 ml of 0.250 m. The key to solving this problem is to. Hess’s law tells us the overall enthalpy change for both routes will be the same. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: This is one version of the first. Enthalpy Of Combustion Hess Law.
From www.science-revision.co.uk
Hess's Law Enthalpy Of Combustion Hess Law We can apply the data from the experimental enthalpies of combustion in. Hess’s law tells us the overall enthalpy change for both routes will be the same. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: According to hess’s law, the enthalpy change of the reaction will equal the sum. Enthalpy Of Combustion Hess Law.
From general.chemistrysteps.com
Gibbs Free Energy and Hess's Law Chemistry Steps Enthalpy Of Combustion Hess Law Standard enthalpy changes of combustion, δh° c are relatively easy to measure. Explain hess’s law and use it to compute reaction enthalpies standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1. For benzene, carbon and hydrogen, these are: What is the enthalpy of combustion per mole of methane under these conditions? How much heat is produced when. Enthalpy Of Combustion Hess Law.
From www.youtube.com
Hess's Law and Enthalpy of Combustion, Paper 1 & 2 AQA A level Enthalpy Of Combustion Hess Law We can use enthalpies of combustion data to find the enthalpy changes for combustion reactions in the cycle. Hess’s law tells us the overall enthalpy change for both routes will be the same. The key to solving this problem is to. According to hess’s law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of. Enthalpy Of Combustion Hess Law.
From www.youtube.com
Bond Enthalpy of Rxn Hess Law for Combustion of Ethane YouTube Enthalpy Of Combustion Hess Law How much heat is produced when 100 ml of 0.250 m. We can apply the data from the experimental enthalpies of combustion in. It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: This is one version of the first law of thermodynamics, and it shows that the internal energy of. Enthalpy Of Combustion Hess Law.
From www.youtube.com
(Video 2) 5.2 Hess's Law Enthalpy change of combustion energy cycles Enthalpy Of Combustion Hess Law Explain hess’s law and use it to compute reaction enthalpies standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1. According to hess’s law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of the steps. We can apply the data from the experimental enthalpies of combustion in. We can use enthalpies of. Enthalpy Of Combustion Hess Law.
From www.slideserve.com
PPT Thermochemisty (Enthalpy) and Hess’s Law PowerPoint Presentation Enthalpy Of Combustion Hess Law It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: Standard enthalpy changes of combustion, δh° c are relatively easy to measure. We can apply the data from the experimental enthalpies of combustion in. What is the enthalpy of combustion per mole of methane under these conditions? Explain hess’s law and. Enthalpy Of Combustion Hess Law.
From www.chemistrystudent.com
Hess' Law and Hess Cycles (ALevel) ChemistryStudent Enthalpy Of Combustion Hess Law How much heat is produced when 100 ml of 0.250 m. We can use enthalpies of combustion data to find the enthalpy changes for combustion reactions in the cycle. Standard enthalpy changes of combustion, δh° c are relatively easy to measure. According to hess’s law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of. Enthalpy Of Combustion Hess Law.
From www.scribd.com
5.1. HESS'S LAW.ppt Combustion Enthalpy Free 30day Trial Scribd Enthalpy Of Combustion Hess Law How much heat is produced when 100 ml of 0.250 m. Write down the enthalpy change. For benzene, carbon and hydrogen, these are: This is one version of the first law of thermodynamics, and it shows that the internal energy of a system changes through heat flow. What is the enthalpy of combustion per mole of methane under these conditions?. Enthalpy Of Combustion Hess Law.
From study.com
Using Hess's Law to Calculate the Enthalpy of a Reaction Chemistry Enthalpy Of Combustion Hess Law It is so common that the phrase standard enthalpy of combustion is used alot and is given this symbol: For benzene, carbon and hydrogen, these are: We can apply the data from the experimental enthalpies of combustion in. This is one version of the first law of thermodynamics, and it shows that the internal energy of a system changes through. Enthalpy Of Combustion Hess Law.
From www.chemistrystudent.com
Hess' Law and Hess Cycles (ALevel) ChemistryStudent Enthalpy Of Combustion Hess Law This is one version of the first law of thermodynamics, and it shows that the internal energy of a system changes through heat flow. Explain hess’s law and use it to compute reaction enthalpies standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1. Write down the enthalpy change. What is the enthalpy of combustion per mole of. Enthalpy Of Combustion Hess Law.
From www.sliderbase.com
Hess's Law Presentation Chemistry Enthalpy Of Combustion Hess Law Standard enthalpy changes of combustion, δh° c are relatively easy to measure. For benzene, carbon and hydrogen, these are: We can apply the data from the experimental enthalpies of combustion in. According to hess’s law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of the steps. According to hess’s law, the enthalpy change of. Enthalpy Of Combustion Hess Law.