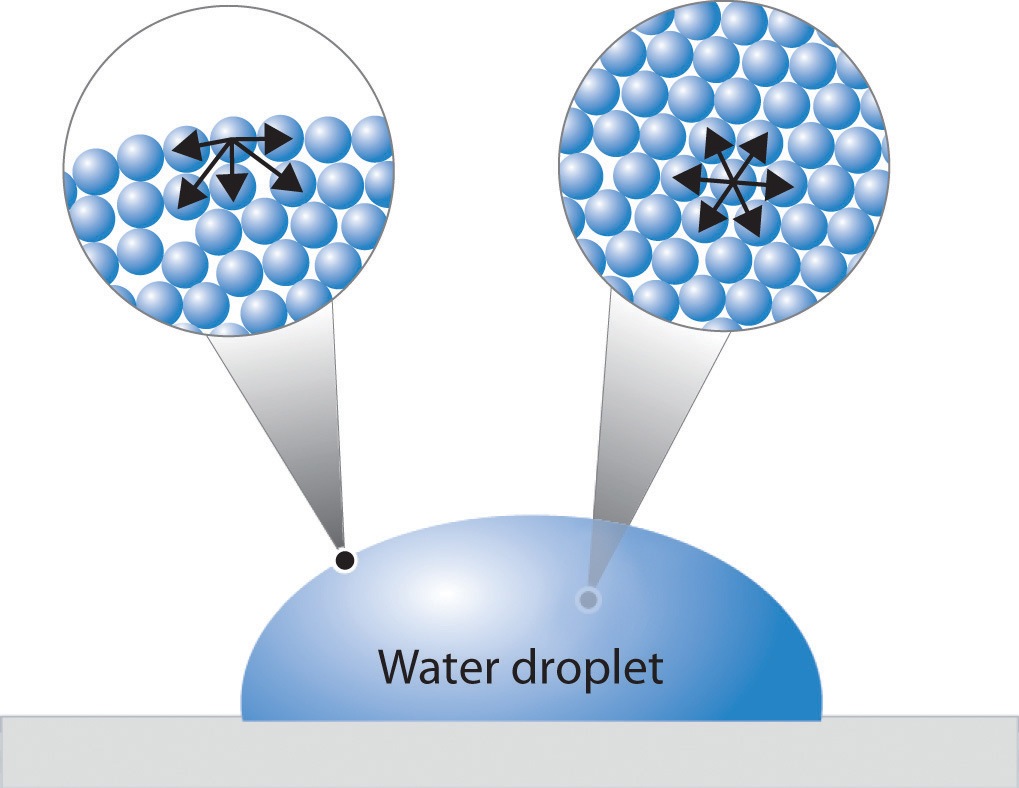
How Does Surface Tension Force The Water To Curve . In comparison, organic liquids, such as benzene and alcohols,. (a) mercury is suppressed in a glass tube because its contact angle is greater than \(90^o\). The cohesive forces between liquid molecules. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension thus flattens the curved liquid surface in a capillary tube. This general effect is called surface tension. Attractive forces between molecules of different types are called adhesive forces. This results in a downward force in mercury and an upward force in water, as seen in figure. — water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). — surface tension in liquid water resists an external force because of cohesive. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\).
from chem.libretexts.org
The cohesive forces between liquid molecules. — water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. (a) mercury is suppressed in a glass tube because its contact angle is greater than \(90^o\). This general effect is called surface tension. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\). — surface tension in liquid water resists an external force because of cohesive. Attractive forces between molecules of different types are called adhesive forces. surface tension thus flattens the curved liquid surface in a capillary tube. In comparison, organic liquids, such as benzene and alcohols,.
Surface Tension Chemistry LibreTexts
How Does Surface Tension Force The Water To Curve The cohesive forces between liquid molecules. This results in a downward force in mercury and an upward force in water, as seen in figure. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Attractive forces between molecules of different types are called adhesive forces. — water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). The cohesive forces between liquid molecules. In comparison, organic liquids, such as benzene and alcohols,. (a) mercury is suppressed in a glass tube because its contact angle is greater than \(90^o\). surface tension thus flattens the curved liquid surface in a capillary tube. — surface tension in liquid water resists an external force because of cohesive. This general effect is called surface tension. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\).
From errantscience.com
Water surface tension ErrantScience How Does Surface Tension Force The Water To Curve Attractive forces between molecules of different types are called adhesive forces. (a) mercury is suppressed in a glass tube because its contact angle is greater than \(90^o\). Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. — water has a surface tension of 0.07275 joule per square metre at 20. How Does Surface Tension Force The Water To Curve.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) How Does Surface Tension Force The Water To Curve surface tension thus flattens the curved liquid surface in a capillary tube. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. In comparison, organic liquids, such as benzene and alcohols,. (a) mercury is suppressed in a glass tube because its contact angle is greater than \(90^o\). This results in a. How Does Surface Tension Force The Water To Curve.
From www.researchgate.net
Surface tension forces and the contact angle applied to the composition How Does Surface Tension Force The Water To Curve — water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). The cohesive forces between liquid molecules. This results in a downward force in mercury and an upward force in water, as seen in figure. Attractive forces between molecules of different types are called adhesive forces. Cohesive forces between molecules cause the surface. How Does Surface Tension Force The Water To Curve.
From guidemanualspyglasses.z14.web.core.windows.net
How To Explain Surface Tension How Does Surface Tension Force The Water To Curve Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. This results in a downward force in mercury and an upward force in water, as seen in figure. surface tension thus flattens the curved liquid surface in a capillary tube. In comparison, organic liquids, such as benzene and alcohols,. Attractive forces. How Does Surface Tension Force The Water To Curve.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and How Does Surface Tension Force The Water To Curve Attractive forces between molecules of different types are called adhesive forces. In comparison, organic liquids, such as benzene and alcohols,. (a) mercury is suppressed in a glass tube because its contact angle is greater than \(90^o\). Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. — water has a surface. How Does Surface Tension Force The Water To Curve.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector How Does Surface Tension Force The Water To Curve The cohesive forces between liquid molecules. (a) mercury is suppressed in a glass tube because its contact angle is greater than \(90^o\). surface tension thus flattens the curved liquid surface in a capillary tube. This general effect is called surface tension. — water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f).. How Does Surface Tension Force The Water To Curve.
From www.vrogue.co
What Are The Importances Of Surface Tension And Visco vrogue.co How Does Surface Tension Force The Water To Curve surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\). This general effect is called surface tension. surface tension thus flattens the curved liquid surface in a capillary tube. The cohesive forces between liquid molecules. (a) mercury is suppressed in a glass tube because its. How Does Surface Tension Force The Water To Curve.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces How Does Surface Tension Force The Water To Curve The cohesive forces between liquid molecules. (a) mercury is suppressed in a glass tube because its contact angle is greater than \(90^o\). This general effect is called surface tension. Attractive forces between molecules of different types are called adhesive forces. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. This results. How Does Surface Tension Force The Water To Curve.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 How Does Surface Tension Force The Water To Curve Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. — surface tension in liquid water resists an external force because of cohesive. In comparison, organic liquids, such as benzene and alcohols,. surface tension of water can cause things to float which are denser than water, allowing organisms to literally. How Does Surface Tension Force The Water To Curve.
From www.biolinscientific.com
Surface tension of water Why is it so high? How Does Surface Tension Force The Water To Curve The cohesive forces between liquid molecules. (a) mercury is suppressed in a glass tube because its contact angle is greater than \(90^o\). Attractive forces between molecules of different types are called adhesive forces. — surface tension in liquid water resists an external force because of cohesive. — water has a surface tension of 0.07275 joule per square metre. How Does Surface Tension Force The Water To Curve.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL How Does Surface Tension Force The Water To Curve — surface tension in liquid water resists an external force because of cohesive. In comparison, organic liquids, such as benzene and alcohols,. — water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension of water can cause things to float which are denser than water, allowing organisms to literally. How Does Surface Tension Force The Water To Curve.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs How Does Surface Tension Force The Water To Curve surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\). In comparison, organic liquids, such as benzene and alcohols,. (a) mercury is suppressed in a glass tube because its contact angle is greater than \(90^o\). Cohesive forces between molecules cause the surface of a liquid to. How Does Surface Tension Force The Water To Curve.
From study.com
Surface Tension Definition, Calculation & Examples Video & Lesson How Does Surface Tension Force The Water To Curve Attractive forces between molecules of different types are called adhesive forces. This results in a downward force in mercury and an upward force in water, as seen in figure. The cohesive forces between liquid molecules. — surface tension in liquid water resists an external force because of cohesive. surface tension of water can cause things to float which. How Does Surface Tension Force The Water To Curve.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids and Solids PowerPoint How Does Surface Tension Force The Water To Curve This general effect is called surface tension. The cohesive forces between liquid molecules. (a) mercury is suppressed in a glass tube because its contact angle is greater than \(90^o\). Attractive forces between molecules of different types are called adhesive forces. This results in a downward force in mercury and an upward force in water, as seen in figure. surface. How Does Surface Tension Force The Water To Curve.
From exoorzvyy.blob.core.windows.net
Surface Tension Of Water Lesson Plan at Sabrina Nickerson blog How Does Surface Tension Force The Water To Curve In comparison, organic liquids, such as benzene and alcohols,. surface tension thus flattens the curved liquid surface in a capillary tube. This results in a downward force in mercury and an upward force in water, as seen in figure. — water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). The cohesive. How Does Surface Tension Force The Water To Curve.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts How Does Surface Tension Force The Water To Curve Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. — surface tension in liquid water resists an external force because of cohesive. surface tension thus flattens the curved liquid surface in a capillary tube. (a) mercury is suppressed in a glass tube because its contact angle is greater than. How Does Surface Tension Force The Water To Curve.
From sophisticatedthoughtscom.wordpress.com
The Science Behind Bubbles (1/3) Sophisticated Thoughts How Does Surface Tension Force The Water To Curve surface tension thus flattens the curved liquid surface in a capillary tube. The cohesive forces between liquid molecules. — surface tension in liquid water resists an external force because of cohesive. In comparison, organic liquids, such as benzene and alcohols,. surface tension of water can cause things to float which are denser than water, allowing organisms to. How Does Surface Tension Force The Water To Curve.
From www.slideserve.com
PPT FLUID PROPERTIES Chapter 2 PowerPoint Presentation, free download How Does Surface Tension Force The Water To Curve — surface tension in liquid water resists an external force because of cohesive. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. In comparison, organic liquids, such as benzene and alcohols,. surface tension thus flattens the curved liquid surface in a capillary tube. The cohesive forces between liquid molecules.. How Does Surface Tension Force The Water To Curve.
From circuitdbseriatim.z13.web.core.windows.net
Diagram Of Surface Tension How Does Surface Tension Force The Water To Curve Attractive forces between molecules of different types are called adhesive forces. This results in a downward force in mercury and an upward force in water, as seen in figure. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\). Cohesive forces between molecules cause the surface. How Does Surface Tension Force The Water To Curve.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance How Does Surface Tension Force The Water To Curve — water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Attractive forces between molecules of different types are called adhesive forces. In comparison, organic liquids, such as benzene and alcohols,. The cohesive forces between liquid molecules. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible. How Does Surface Tension Force The Water To Curve.
From www.slideserve.com
PPT Water and Aqueous Systems PowerPoint Presentation ID565121 How Does Surface Tension Force The Water To Curve Attractive forces between molecules of different types are called adhesive forces. surface tension thus flattens the curved liquid surface in a capillary tube. (a) mercury is suppressed in a glass tube because its contact angle is greater than \(90^o\). This results in a downward force in mercury and an upward force in water, as seen in figure. —. How Does Surface Tension Force The Water To Curve.
From courses.lumenlearning.com
Cohesion and Adhesion in Liquids Surface Tension and Capillary Action How Does Surface Tension Force The Water To Curve This general effect is called surface tension. In comparison, organic liquids, such as benzene and alcohols,. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\). (a) mercury is suppressed in a glass tube because its contact angle is greater than \(90^o\). Attractive forces between molecules. How Does Surface Tension Force The Water To Curve.
From studyschoollifebelts.z21.web.core.windows.net
How To Explain Surface Tension How Does Surface Tension Force The Water To Curve This general effect is called surface tension. (a) mercury is suppressed in a glass tube because its contact angle is greater than \(90^o\). Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. In comparison, organic liquids, such as benzene and alcohols,. — water has a surface tension of 0.07275 joule. How Does Surface Tension Force The Water To Curve.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint How Does Surface Tension Force The Water To Curve Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Attractive forces between molecules of different types are called adhesive forces. This general effect is called surface tension. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\).. How Does Surface Tension Force The Water To Curve.
From dxosoutiz.blob.core.windows.net
How To Calculate Water Surface Tension at Dana Zheng blog How Does Surface Tension Force The Water To Curve The cohesive forces between liquid molecules. Attractive forces between molecules of different types are called adhesive forces. This results in a downward force in mercury and an upward force in water, as seen in figure. This general effect is called surface tension. surface tension thus flattens the curved liquid surface in a capillary tube. (a) mercury is suppressed in. How Does Surface Tension Force The Water To Curve.
From www.youtube.com
Chemistry 8.2b Properties of Liquids Surface Tension and Capillary How Does Surface Tension Force The Water To Curve This results in a downward force in mercury and an upward force in water, as seen in figure. Attractive forces between molecules of different types are called adhesive forces. (a) mercury is suppressed in a glass tube because its contact angle is greater than \(90^o\). — water has a surface tension of 0.07275 joule per square metre at 20. How Does Surface Tension Force The Water To Curve.
From studylibrarytoweled.z13.web.core.windows.net
What Has Surface Tension How Does Surface Tension Force The Water To Curve surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\). In comparison, organic liquids, such as benzene and alcohols,. The cohesive forces between liquid molecules. This results in a downward force in mercury and an upward force in water, as seen in figure. — surface. How Does Surface Tension Force The Water To Curve.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy How Does Surface Tension Force The Water To Curve In comparison, organic liquids, such as benzene and alcohols,. — water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension thus flattens the curved liquid surface in a capillary tube. This results in a downward force in mercury and an upward force in water, as seen in figure. Attractive forces. How Does Surface Tension Force The Water To Curve.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs How Does Surface Tension Force The Water To Curve surface tension thus flattens the curved liquid surface in a capillary tube. (a) mercury is suppressed in a glass tube because its contact angle is greater than \(90^o\). This results in a downward force in mercury and an upward force in water, as seen in figure. Cohesive forces between molecules cause the surface of a liquid to contract to. How Does Surface Tension Force The Water To Curve.
From courses.lumenlearning.com
Surface Tension Introduction to Chemistry How Does Surface Tension Force The Water To Curve — surface tension in liquid water resists an external force because of cohesive. — water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Attractive forces between molecules of different types are called adhesive forces. In comparison, organic liquids, such as benzene and alcohols,. surface tension thus flattens the curved liquid. How Does Surface Tension Force The Water To Curve.
From www.slideserve.com
PPT Chapter 2 Properties of Fluids PowerPoint Presentation, free How Does Surface Tension Force The Water To Curve This general effect is called surface tension. Attractive forces between molecules of different types are called adhesive forces. (a) mercury is suppressed in a glass tube because its contact angle is greater than \(90^o\). The cohesive forces between liquid molecules. — surface tension in liquid water resists an external force because of cohesive. surface tension thus flattens the. How Does Surface Tension Force The Water To Curve.
From byjus.com
Explain the surface tension phenomenon with examples. How Does Surface Tension Force The Water To Curve — water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\). (a) mercury is suppressed in a glass tube because its contact angle is greater than \(90^o\). This general. How Does Surface Tension Force The Water To Curve.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit How Does Surface Tension Force The Water To Curve The cohesive forces between liquid molecules. This results in a downward force in mercury and an upward force in water, as seen in figure. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\). — surface tension in liquid water resists an external force because. How Does Surface Tension Force The Water To Curve.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog How Does Surface Tension Force The Water To Curve — water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). This results in a downward force in mercury and an upward force in water, as seen in figure. (a) mercury is suppressed in a glass tube because its contact angle is greater than \(90^o\). The cohesive forces between liquid molecules. surface. How Does Surface Tension Force The Water To Curve.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 How Does Surface Tension Force The Water To Curve This general effect is called surface tension. — surface tension in liquid water resists an external force because of cohesive. This results in a downward force in mercury and an upward force in water, as seen in figure. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on. How Does Surface Tension Force The Water To Curve.