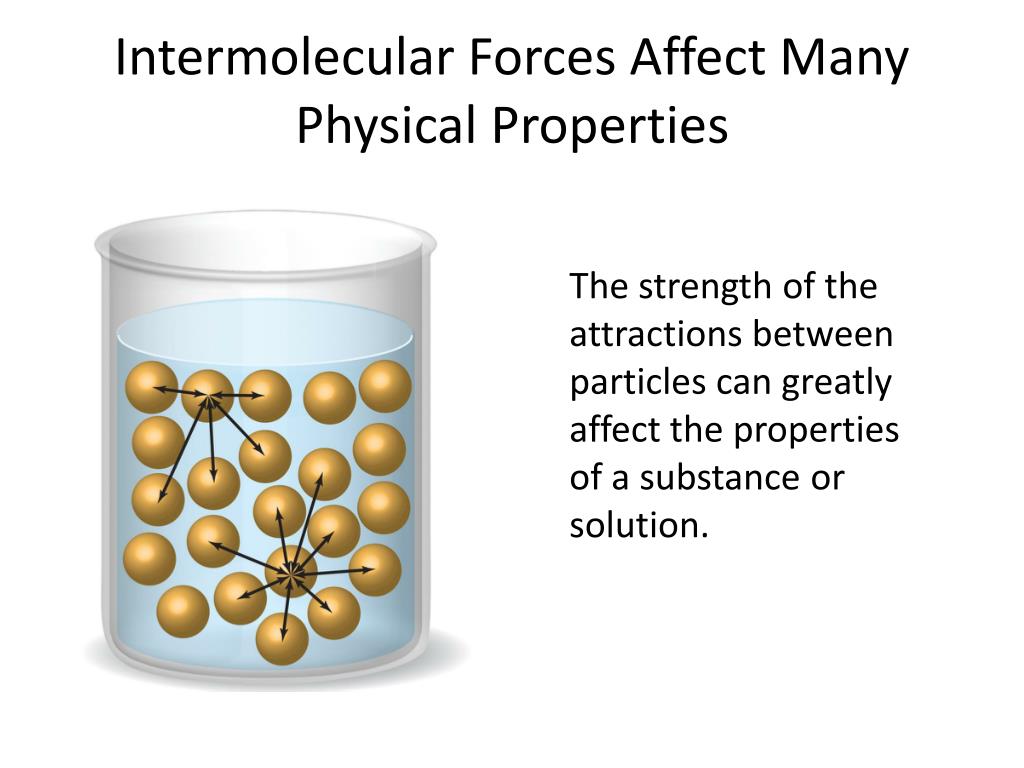
How Does Surface Tension Affect Intermolecular Forces . intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. molecules in the liquid state experience strong intermolecular attractive forces. The liquid film exerts a force on the movable wire in an attempt to. the answer lies in a property called surface tension, which depends on intermolecular forces. surface tension is caused by the effects of intermolecular forces at the interface. Surface tension depends on the nature of the liquid, the surrounding. liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest. figure 11.26 shows one way to measure surface tension. When those forces are between like molecules, they are referred to as cohesive forces.
from www.slideserve.com
the answer lies in a property called surface tension, which depends on intermolecular forces. molecules in the liquid state experience strong intermolecular attractive forces. surface tension is caused by the effects of intermolecular forces at the interface. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest. figure 11.26 shows one way to measure surface tension. Surface tension depends on the nature of the liquid, the surrounding. When those forces are between like molecules, they are referred to as cohesive forces. intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical. The liquid film exerts a force on the movable wire in an attempt to.
PPT Chapter 9 Intermolecular Forces, Liquids, and Solids PowerPoint
How Does Surface Tension Affect Intermolecular Forces intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical. figure 11.26 shows one way to measure surface tension. When those forces are between like molecules, they are referred to as cohesive forces. surface tension is caused by the effects of intermolecular forces at the interface. The liquid film exerts a force on the movable wire in an attempt to. molecules in the liquid state experience strong intermolecular attractive forces. liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest. intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical. Surface tension depends on the nature of the liquid, the surrounding. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. the answer lies in a property called surface tension, which depends on intermolecular forces.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint How Does Surface Tension Affect Intermolecular Forces molecules in the liquid state experience strong intermolecular attractive forces. The liquid film exerts a force on the movable wire in an attempt to. figure 11.26 shows one way to measure surface tension. surface tension is caused by the effects of intermolecular forces at the interface. the answer lies in a property called surface tension, which. How Does Surface Tension Affect Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID How Does Surface Tension Affect Intermolecular Forces the answer lies in a property called surface tension, which depends on intermolecular forces. intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. liquids that have. How Does Surface Tension Affect Intermolecular Forces.
From www.youtube.com
Surface tension and capillary action Intermolecular Forces meriSTEM How Does Surface Tension Affect Intermolecular Forces intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical. Surface tension depends on the nature of the liquid, the surrounding. figure 11.26 shows one way to measure surface tension. The liquid film exerts a force on the movable wire in an attempt to. surface tension is the. How Does Surface Tension Affect Intermolecular Forces.
From www.shutterstock.com
Surface Tension Demonstrated By Intermolecular Interaction Stock How Does Surface Tension Affect Intermolecular Forces liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest. When those forces are between like molecules, they are referred to as cohesive forces. surface tension is caused by the effects of intermolecular forces at the interface. Surface tension depends on the nature of the liquid, the surrounding. the answer lies in. How Does Surface Tension Affect Intermolecular Forces.
From jsmithmoore.com
Intermolecular forces How Does Surface Tension Affect Intermolecular Forces When those forces are between like molecules, they are referred to as cohesive forces. intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical. surface tension is caused by the effects of intermolecular forces at the interface. liquids that have strong intermolecular forces, like the hydrogen bonding in. How Does Surface Tension Affect Intermolecular Forces.
From 88guru.com
Viscosity And Surface Tension Definition, Applications and FAQ's 88Guru How Does Surface Tension Affect Intermolecular Forces The liquid film exerts a force on the movable wire in an attempt to. Surface tension depends on the nature of the liquid, the surrounding. When those forces are between like molecules, they are referred to as cohesive forces. figure 11.26 shows one way to measure surface tension. surface tension is caused by the effects of intermolecular forces. How Does Surface Tension Affect Intermolecular Forces.
From www.youtube.com
Intermolecular Forces Trends Melting & Boiling Point, Viscosity How Does Surface Tension Affect Intermolecular Forces The liquid film exerts a force on the movable wire in an attempt to. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical. the answer lies in. How Does Surface Tension Affect Intermolecular Forces.
From www.slideserve.com
PPT Chapter 12 Intermolecular Attractions and the Properties of How Does Surface Tension Affect Intermolecular Forces molecules in the liquid state experience strong intermolecular attractive forces. When those forces are between like molecules, they are referred to as cohesive forces. figure 11.26 shows one way to measure surface tension. surface tension is caused by the effects of intermolecular forces at the interface. The liquid film exerts a force on the movable wire in. How Does Surface Tension Affect Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces, Liquids, and Solids PowerPoint How Does Surface Tension Affect Intermolecular Forces the answer lies in a property called surface tension, which depends on intermolecular forces. surface tension is caused by the effects of intermolecular forces at the interface. figure 11.26 shows one way to measure surface tension. Surface tension depends on the nature of the liquid, the surrounding. surface tension is the energy, or work, required to. How Does Surface Tension Affect Intermolecular Forces.
From fyozfaljz.blob.core.windows.net
How Does Surface Tension Affect Humans at Andrew Rex blog How Does Surface Tension Affect Intermolecular Forces molecules in the liquid state experience strong intermolecular attractive forces. Surface tension depends on the nature of the liquid, the surrounding. figure 11.26 shows one way to measure surface tension. surface tension is caused by the effects of intermolecular forces at the interface. surface tension is the energy, or work, required to increase the surface area. How Does Surface Tension Affect Intermolecular Forces.
From slideplayer.com
Intermolecular forces ppt download How Does Surface Tension Affect Intermolecular Forces molecules in the liquid state experience strong intermolecular attractive forces. the answer lies in a property called surface tension, which depends on intermolecular forces. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. When those forces are between like molecules, they are referred to as cohesive. How Does Surface Tension Affect Intermolecular Forces.
From byjus.com
Explain the surface tension phenomenon with examples. How Does Surface Tension Affect Intermolecular Forces The liquid film exerts a force on the movable wire in an attempt to. molecules in the liquid state experience strong intermolecular attractive forces. figure 11.26 shows one way to measure surface tension. Surface tension depends on the nature of the liquid, the surrounding. When those forces are between like molecules, they are referred to as cohesive forces.. How Does Surface Tension Affect Intermolecular Forces.
From slideplayer.com
Intermolecular forces ppt download How Does Surface Tension Affect Intermolecular Forces Surface tension depends on the nature of the liquid, the surrounding. surface tension is caused by the effects of intermolecular forces at the interface. When those forces are between like molecules, they are referred to as cohesive forces. liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest. the answer lies in. How Does Surface Tension Affect Intermolecular Forces.
From chem.libretexts.org
11.4 Intermolecular Forces in Action Surface Tension, Viscosity, and How Does Surface Tension Affect Intermolecular Forces surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is caused by the effects of intermolecular forces at the interface. molecules in the liquid state experience strong intermolecular attractive forces. liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit. How Does Surface Tension Affect Intermolecular Forces.
From www.slideserve.com
PPT Covalent Bonding & Intermolecular Forces PowerPoint Presentation How Does Surface Tension Affect Intermolecular Forces the answer lies in a property called surface tension, which depends on intermolecular forces. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension depends on the nature of the liquid, the surrounding. molecules in the liquid state experience strong intermolecular attractive forces. surface. How Does Surface Tension Affect Intermolecular Forces.
From www.slideserve.com
PPT Polarity and Intermolecular Forces PowerPoint Presentation, free How Does Surface Tension Affect Intermolecular Forces surface tension is caused by the effects of intermolecular forces at the interface. the answer lies in a property called surface tension, which depends on intermolecular forces. figure 11.26 shows one way to measure surface tension. The liquid film exerts a force on the movable wire in an attempt to. When those forces are between like molecules,. How Does Surface Tension Affect Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces, Liquids, and Solids PowerPoint How Does Surface Tension Affect Intermolecular Forces liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest. molecules in the liquid state experience strong intermolecular attractive forces. When those forces are between like molecules, they are referred to as cohesive forces. The liquid film exerts a force on the movable wire in an attempt to. the answer lies in. How Does Surface Tension Affect Intermolecular Forces.
From slideplayer.com
Intermolecular Forces ppt download How Does Surface Tension Affect Intermolecular Forces intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. the answer lies in a property called surface tension, which depends on intermolecular forces. When those forces are. How Does Surface Tension Affect Intermolecular Forces.
From www.slideserve.com
PPT Chapter 9 Intermolecular Forces, Liquids, and Solids PowerPoint How Does Surface Tension Affect Intermolecular Forces Surface tension depends on the nature of the liquid, the surrounding. The liquid film exerts a force on the movable wire in an attempt to. When those forces are between like molecules, they are referred to as cohesive forces. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.. How Does Surface Tension Affect Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint How Does Surface Tension Affect Intermolecular Forces intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. figure 11.26 shows one way to measure surface tension. liquids that have strong intermolecular forces, like the. How Does Surface Tension Affect Intermolecular Forces.
From www.researchgate.net
2. Intermolecular forces causing the surface tension phenomena (A) and How Does Surface Tension Affect Intermolecular Forces molecules in the liquid state experience strong intermolecular attractive forces. When those forces are between like molecules, they are referred to as cohesive forces. intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical. liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the. How Does Surface Tension Affect Intermolecular Forces.
From slideplayer.com
Chapter 11 Intermolecular Attractive Forces, Liquids, and Solids ppt How Does Surface Tension Affect Intermolecular Forces figure 11.26 shows one way to measure surface tension. Surface tension depends on the nature of the liquid, the surrounding. When those forces are between like molecules, they are referred to as cohesive forces. intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical. The liquid film exerts a. How Does Surface Tension Affect Intermolecular Forces.
From www.slideserve.com
PPT Implication of Intermolecular Forces PowerPoint Presentation How Does Surface Tension Affect Intermolecular Forces When those forces are between like molecules, they are referred to as cohesive forces. liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. molecules in the liquid state experience strong intermolecular. How Does Surface Tension Affect Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids and Solids PowerPoint How Does Surface Tension Affect Intermolecular Forces the answer lies in a property called surface tension, which depends on intermolecular forces. When those forces are between like molecules, they are referred to as cohesive forces. The liquid film exerts a force on the movable wire in an attempt to. surface tension is the energy, or work, required to increase the surface area of a liquid. How Does Surface Tension Affect Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Liquids and Intermolecular Forces PowerPoint How Does Surface Tension Affect Intermolecular Forces figure 11.26 shows one way to measure surface tension. The liquid film exerts a force on the movable wire in an attempt to. Surface tension depends on the nature of the liquid, the surrounding. intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical. liquids that have strong. How Does Surface Tension Affect Intermolecular Forces.
From slideplayer.com
States of Matter. ppt download How Does Surface Tension Affect Intermolecular Forces figure 11.26 shows one way to measure surface tension. molecules in the liquid state experience strong intermolecular attractive forces. the answer lies in a property called surface tension, which depends on intermolecular forces. The liquid film exerts a force on the movable wire in an attempt to. Surface tension depends on the nature of the liquid, the. How Does Surface Tension Affect Intermolecular Forces.
From www.slideserve.com
PPT States of Matter and Intermolecular Forces PowerPoint How Does Surface Tension Affect Intermolecular Forces surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. the answer lies in a property called surface tension, which depends on intermolecular forces. intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical. figure 11.26 shows. How Does Surface Tension Affect Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download How Does Surface Tension Affect Intermolecular Forces molecules in the liquid state experience strong intermolecular attractive forces. liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest. figure 11.26 shows one way to measure surface tension. When those forces are between like molecules, they are referred to as cohesive forces. intermolecular forces have varying strengths, and the strength. How Does Surface Tension Affect Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID How Does Surface Tension Affect Intermolecular Forces intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical. Surface tension depends on the nature of the liquid, the surrounding. liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest. the answer lies in a property called surface tension, which depends on. How Does Surface Tension Affect Intermolecular Forces.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs How Does Surface Tension Affect Intermolecular Forces surface tension is caused by the effects of intermolecular forces at the interface. Surface tension depends on the nature of the liquid, the surrounding. liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest. intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s. How Does Surface Tension Affect Intermolecular Forces.
From www.slideserve.com
PPT Chapter 9 Intermolecular Forces, Liquids, and Solids PowerPoint How Does Surface Tension Affect Intermolecular Forces surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is caused by the effects of intermolecular forces at the interface. When those forces are between like molecules, they are referred to as cohesive forces. Surface tension depends on the nature of the liquid, the surrounding.. How Does Surface Tension Affect Intermolecular Forces.
From www.youtube.com
12.3 Intermolecular Forces in Action Surface Tension & Viscosity YouTube How Does Surface Tension Affect Intermolecular Forces molecules in the liquid state experience strong intermolecular attractive forces. the answer lies in a property called surface tension, which depends on intermolecular forces. intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical. surface tension is caused by the effects of intermolecular forces at the interface.. How Does Surface Tension Affect Intermolecular Forces.
From www.slideserve.com
PPT AP Chapter 11 PowerPoint Presentation, free download ID2275798 How Does Surface Tension Affect Intermolecular Forces surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is caused by the effects of intermolecular forces at the interface. liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest. figure 11.26 shows one way to measure surface. How Does Surface Tension Affect Intermolecular Forces.
From www.slideserve.com
PPT Chapter 12 Intermolecular Attractions and the Properties of How Does Surface Tension Affect Intermolecular Forces Surface tension depends on the nature of the liquid, the surrounding. molecules in the liquid state experience strong intermolecular attractive forces. surface tension is caused by the effects of intermolecular forces at the interface. liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest. the answer lies in a property called. How Does Surface Tension Affect Intermolecular Forces.
From www.slideserve.com
PPT Chapter 12 Intermolecular Forces PowerPoint Presentation, free How Does Surface Tension Affect Intermolecular Forces The liquid film exerts a force on the movable wire in an attempt to. liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest. the answer lies in a property called surface tension, which depends on intermolecular forces. figure 11.26 shows one way to measure surface tension. When those forces are between. How Does Surface Tension Affect Intermolecular Forces.