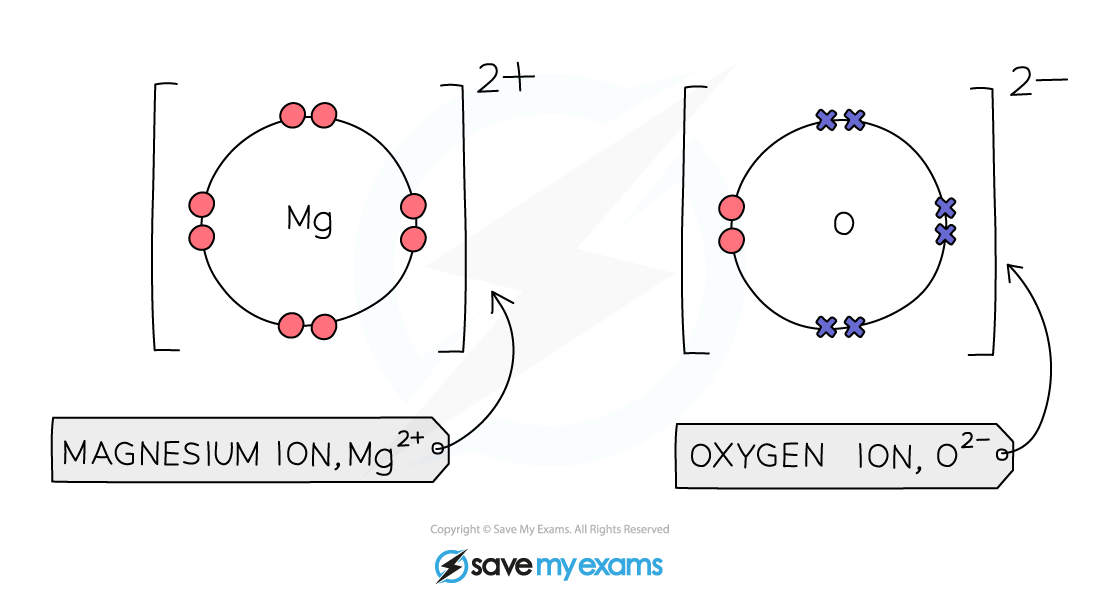
Magnesium Atoms Have Two Electrons . The electron configuration of magnesium ion(mg +2) is 1s 2 2s 2 2p 6. Learn the definition and examples of valence electrons for main. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. Also, the electron configuration of mg is 1s² 2s²2p⁶ 3s² or [ne]3s². Magnesium donates two electrons to. When a magnesium atom reacts with an oxygen atom, magnesium gives up its two valence electrons and becomes a positively charged ion, i.e. Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. The first two electrons are found in the \(n=1\) energy level, the next eight. An element in group 2 has two valence electrons. Find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. Learn how magnesium and oxygen atoms react to form magnesium oxide, a giant ionic lattice structure.
from www.linstitute.net
The first two electrons are found in the \(n=1\) energy level, the next eight. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. Magnesium donates two electrons to. Find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. Learn the definition and examples of valence electrons for main. An element in group 2 has two valence electrons. Learn how magnesium and oxygen atoms react to form magnesium oxide, a giant ionic lattice structure. When a magnesium atom reacts with an oxygen atom, magnesium gives up its two valence electrons and becomes a positively charged ion, i.e. The electron configuration of magnesium ion(mg +2) is 1s 2 2s 2 2p 6. Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons.
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:1.6 4 Ionic Bonds Dot
Magnesium Atoms Have Two Electrons When a magnesium atom reacts with an oxygen atom, magnesium gives up its two valence electrons and becomes a positively charged ion, i.e. The electron configuration of magnesium ion(mg +2) is 1s 2 2s 2 2p 6. When a magnesium atom reacts with an oxygen atom, magnesium gives up its two valence electrons and becomes a positively charged ion, i.e. An element in group 2 has two valence electrons. Also, the electron configuration of mg is 1s² 2s²2p⁶ 3s² or [ne]3s². Learn the definition and examples of valence electrons for main. The first two electrons are found in the \(n=1\) energy level, the next eight. Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. Learn how magnesium and oxygen atoms react to form magnesium oxide, a giant ionic lattice structure. Find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. Magnesium donates two electrons to.
From slideplayer.com
The Periodic Table and How it is Organized. ppt download Magnesium Atoms Have Two Electrons Also, the electron configuration of mg is 1s² 2s²2p⁶ 3s² or [ne]3s². Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. The first two electrons are found in the \(n=1\) energy level, the next eight. Learn the definition and examples of valence electrons for main. An element in group 2 has two. Magnesium Atoms Have Two Electrons.
From utedzz.blogspot.com
Periodic Table Magnesium Atomic Number Periodic Table Timeline Magnesium Atoms Have Two Electrons Learn how magnesium and oxygen atoms react to form magnesium oxide, a giant ionic lattice structure. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. When a magnesium atom reacts with an oxygen atom, magnesium gives up its two valence electrons and becomes a positively charged ion, i.e. Learn the. Magnesium Atoms Have Two Electrons.
From www.sciencesfp.com
Electronic structure of matter. San Francisco de Paula, Science Magnesium Atoms Have Two Electrons Learn the definition and examples of valence electrons for main. Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. The electron configuration of magnesium ion(mg +2) is 1s 2 2s 2 2p 6. Find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. The. Magnesium Atoms Have Two Electrons.
From valenceelectrons.com
How Many Protons,Neutrons and Electrons Does Magnesium Have? Magnesium Atoms Have Two Electrons Magnesium donates two electrons to. Learn the definition and examples of valence electrons for main. Learn how magnesium and oxygen atoms react to form magnesium oxide, a giant ionic lattice structure. When a magnesium atom reacts with an oxygen atom, magnesium gives up its two valence electrons and becomes a positively charged ion, i.e. Let's look at the figure below. Magnesium Atoms Have Two Electrons.
From slidetodoc.com
Metal ions Nonmetal ions Positive ion Gain electrons Magnesium Atoms Have Two Electrons Learn how magnesium and oxygen atoms react to form magnesium oxide, a giant ionic lattice structure. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. When a magnesium atom reacts with an oxygen atom, magnesium gives up its two valence electrons and becomes a positively charged ion, i.e. An element. Magnesium Atoms Have Two Electrons.
From enginedatanichered.z21.web.core.windows.net
Magnesium Atom Diagram Magnesium Atoms Have Two Electrons The electron configuration of magnesium ion(mg +2) is 1s 2 2s 2 2p 6. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. An element in group 2 has two valence electrons. Learn how magnesium and oxygen atoms react to form magnesium oxide, a giant ionic lattice structure. Find the. Magnesium Atoms Have Two Electrons.
From www.youtube.com
Atomic Structure (Bohr Model) for Magnesium (Mg) YouTube Magnesium Atoms Have Two Electrons Learn the definition and examples of valence electrons for main. The electron configuration of magnesium ion(mg +2) is 1s 2 2s 2 2p 6. Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom.. Magnesium Atoms Have Two Electrons.
From www.slideserve.com
PPT Isotopes of Magnesium PowerPoint Presentation, free download ID Magnesium Atoms Have Two Electrons When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. The electron configuration of magnesium ion(mg +2) is 1s 2 2s 2 2p 6. Find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. When a magnesium atom reacts with an oxygen atom,. Magnesium Atoms Have Two Electrons.
From commons.wikimedia.org
FileElectron shell 012 Magnesium.svg Wikimedia Commons Magnesium Atoms Have Two Electrons Learn the definition and examples of valence electrons for main. Learn how magnesium and oxygen atoms react to form magnesium oxide, a giant ionic lattice structure. An element in group 2 has two valence electrons. Magnesium donates two electrons to. Also, the electron configuration of mg is 1s² 2s²2p⁶ 3s² or [ne]3s². Find the valence electrons of any element in. Magnesium Atoms Have Two Electrons.
From mungfali.com
Magnesium Orbital Diagram Magnesium Atoms Have Two Electrons Learn how magnesium and oxygen atoms react to form magnesium oxide, a giant ionic lattice structure. Find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. Also, the electron configuration of mg is 1s² 2s²2p⁶. Magnesium Atoms Have Two Electrons.
From www.shutterstock.com
Magnesium Atom. Diagram Representation Of The Element Magnesium Magnesium Atoms Have Two Electrons The first two electrons are found in the \(n=1\) energy level, the next eight. Also, the electron configuration of mg is 1s² 2s²2p⁶ 3s² or [ne]3s². When a magnesium atom reacts with an oxygen atom, magnesium gives up its two valence electrons and becomes a positively charged ion, i.e. An element in group 2 has two valence electrons. Find the. Magnesium Atoms Have Two Electrons.
From valenceelectrons.com
Magnesium(Mg) electron configuration and orbital diagram Magnesium Atoms Have Two Electrons Find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. When a magnesium atom reacts with an oxygen atom, magnesium gives up its two valence electrons and becomes a positively charged ion, i.e. Learn the definition and examples of valence electrons for main. Magnesium donates two electrons to. Also, the electron configuration. Magnesium Atoms Have Two Electrons.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Magnesium Atoms Have Two Electrons The first two electrons are found in the \(n=1\) energy level, the next eight. Learn the definition and examples of valence electrons for main. Learn how magnesium and oxygen atoms react to form magnesium oxide, a giant ionic lattice structure. Find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. Magnesium donates. Magnesium Atoms Have Two Electrons.
From www.thesciencehive.co.uk
Atomic Structure and Electron Configuration (AQA) — the science hive Magnesium Atoms Have Two Electrons The first two electrons are found in the \(n=1\) energy level, the next eight. Also, the electron configuration of mg is 1s² 2s²2p⁶ 3s² or [ne]3s². Learn the definition and examples of valence electrons for main. Find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. Let's look at the figure below. Magnesium Atoms Have Two Electrons.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Magnesium Atoms Have Two Electrons Find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. Also, the electron configuration of mg is 1s² 2s²2p⁶ 3s² or [ne]3s². An element in group 2 has two valence electrons. Learn how magnesium and oxygen atoms react to form magnesium oxide, a giant ionic lattice structure. When we write the configuration. Magnesium Atoms Have Two Electrons.
From www.dreamstime.com
Magnesium Atom, with Mass and Energy Levels. Stock Vector Magnesium Atoms Have Two Electrons The electron configuration of magnesium ion(mg +2) is 1s 2 2s 2 2p 6. Learn the definition and examples of valence electrons for main. When a magnesium atom reacts with an oxygen atom, magnesium gives up its two valence electrons and becomes a positively charged ion, i.e. Also, the electron configuration of mg is 1s² 2s²2p⁶ 3s² or [ne]3s². When. Magnesium Atoms Have Two Electrons.
From www.shutterstock.com
modelo bohr del átomo de magnesio. vector de stock (libre de regalías Magnesium Atoms Have Two Electrons An element in group 2 has two valence electrons. The electron configuration of magnesium ion(mg +2) is 1s 2 2s 2 2p 6. When a magnesium atom reacts with an oxygen atom, magnesium gives up its two valence electrons and becomes a positively charged ion, i.e. Learn the definition and examples of valence electrons for main. Find the valence electrons. Magnesium Atoms Have Two Electrons.
From www.chegg.com
Solved Magnesium atoms have two electrons in their outermost Magnesium Atoms Have Two Electrons The electron configuration of magnesium ion(mg +2) is 1s 2 2s 2 2p 6. Learn the definition and examples of valence electrons for main. Magnesium donates two electrons to. Also, the electron configuration of mg is 1s² 2s²2p⁶ 3s² or [ne]3s². Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. The first. Magnesium Atoms Have Two Electrons.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Atoms Have Two Electrons Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. Find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. When a magnesium atom reacts with. Magnesium Atoms Have Two Electrons.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Atoms Have Two Electrons The first two electrons are found in the \(n=1\) energy level, the next eight. Learn how magnesium and oxygen atoms react to form magnesium oxide, a giant ionic lattice structure. Also, the electron configuration of mg is 1s² 2s²2p⁶ 3s² or [ne]3s². An element in group 2 has two valence electrons. Magnesium donates two electrons to. Find the valence electrons. Magnesium Atoms Have Two Electrons.
From sites.google.com
Atomic Structure Protons, Neutrons and Electrons Mrs. Sanborn's Site Magnesium Atoms Have Two Electrons Learn how magnesium and oxygen atoms react to form magnesium oxide, a giant ionic lattice structure. Learn the definition and examples of valence electrons for main. The first two electrons are found in the \(n=1\) energy level, the next eight. Also, the electron configuration of mg is 1s² 2s²2p⁶ 3s² or [ne]3s². Let's look at the figure below which shows. Magnesium Atoms Have Two Electrons.
From enginedatanichered.z21.web.core.windows.net
Atomic Diagram Of Magnesium Magnesium Atoms Have Two Electrons Also, the electron configuration of mg is 1s² 2s²2p⁶ 3s² or [ne]3s². When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. When a magnesium atom reacts with an oxygen atom, magnesium gives up its two valence electrons and becomes a positively charged ion, i.e. The first two electrons are found. Magnesium Atoms Have Two Electrons.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID5581477 Magnesium Atoms Have Two Electrons The first two electrons are found in the \(n=1\) energy level, the next eight. Learn how magnesium and oxygen atoms react to form magnesium oxide, a giant ionic lattice structure. Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. Also, the electron configuration of mg is 1s² 2s²2p⁶ 3s² or [ne]3s². Magnesium. Magnesium Atoms Have Two Electrons.
From www.alamy.com
3d render of atom structure of magnesium isolated over white background Magnesium Atoms Have Two Electrons Magnesium donates two electrons to. The electron configuration of magnesium ion(mg +2) is 1s 2 2s 2 2p 6. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. When a magnesium atom reacts with an oxygen atom, magnesium gives up its two valence electrons and becomes a positively charged ion,. Magnesium Atoms Have Two Electrons.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Atoms Have Two Electrons Find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. Learn the definition and examples of valence electrons for main. Learn how magnesium and oxygen atoms react to form magnesium oxide, a giant. Magnesium Atoms Have Two Electrons.
From www.youtube.com
Mg Orbital Diagram How to Write the Atomic Orbital Diagram for Magnesium Atoms Have Two Electrons Also, the electron configuration of mg is 1s² 2s²2p⁶ 3s² or [ne]3s². Learn the definition and examples of valence electrons for main. The electron configuration of magnesium ion(mg +2) is 1s 2 2s 2 2p 6. Magnesium donates two electrons to. An element in group 2 has two valence electrons. The first two electrons are found in the \(n=1\) energy. Magnesium Atoms Have Two Electrons.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Atoms Have Two Electrons When a magnesium atom reacts with an oxygen atom, magnesium gives up its two valence electrons and becomes a positively charged ion, i.e. Learn the definition and examples of valence electrons for main. Also, the electron configuration of mg is 1s² 2s²2p⁶ 3s² or [ne]3s². The first two electrons are found in the \(n=1\) energy level, the next eight. Learn. Magnesium Atoms Have Two Electrons.
From ar.inspiredpencil.com
Magnesium Atom Structure Magnesium Atoms Have Two Electrons Magnesium donates two electrons to. Find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. An element in group 2 has two valence electrons. When a magnesium atom reacts with an oxygen atom,. Magnesium Atoms Have Two Electrons.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Magnesium Atoms Have Two Electrons The first two electrons are found in the \(n=1\) energy level, the next eight. Find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. An element in group 2 has two valence electrons.. Magnesium Atoms Have Two Electrons.
From nursehub.com
Electron Shells NurseHub Magnesium Atoms Have Two Electrons Learn how magnesium and oxygen atoms react to form magnesium oxide, a giant ionic lattice structure. Magnesium donates two electrons to. The electron configuration of magnesium ion(mg +2) is 1s 2 2s 2 2p 6. Find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. An element in group 2 has two. Magnesium Atoms Have Two Electrons.
From enginedatanichered.z21.web.core.windows.net
Atomic Diagram Of Magnesium Magnesium Atoms Have Two Electrons The electron configuration of magnesium ion(mg +2) is 1s 2 2s 2 2p 6. Magnesium donates two electrons to. The first two electrons are found in the \(n=1\) energy level, the next eight. When a magnesium atom reacts with an oxygen atom, magnesium gives up its two valence electrons and becomes a positively charged ion, i.e. Learn the definition and. Magnesium Atoms Have Two Electrons.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Atoms Have Two Electrons Magnesium donates two electrons to. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. The first two electrons are found in the \(n=1\) energy level, the next eight. Find the valence electrons of any element in the periodic table, including nitrogen (n) with 5 valence electrons. Learn how magnesium and. Magnesium Atoms Have Two Electrons.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:1.6 4 Ionic Bonds Dot Magnesium Atoms Have Two Electrons When a magnesium atom reacts with an oxygen atom, magnesium gives up its two valence electrons and becomes a positively charged ion, i.e. The electron configuration of magnesium ion(mg +2) is 1s 2 2s 2 2p 6. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. The first two electrons. Magnesium Atoms Have Two Electrons.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Magnesium Atoms Have Two Electrons Learn the definition and examples of valence electrons for main. The first two electrons are found in the \(n=1\) energy level, the next eight. Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. Also, the electron configuration of mg is 1s² 2s²2p⁶ 3s² or [ne]3s². Find the valence electrons of any element. Magnesium Atoms Have Two Electrons.
From www.alamy.com
Magnesium atom diagram concept Stock Vector Image & Art Alamy Magnesium Atoms Have Two Electrons The electron configuration of magnesium ion(mg +2) is 1s 2 2s 2 2p 6. Learn how magnesium and oxygen atoms react to form magnesium oxide, a giant ionic lattice structure. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. Learn the definition and examples of valence electrons for main. Magnesium. Magnesium Atoms Have Two Electrons.