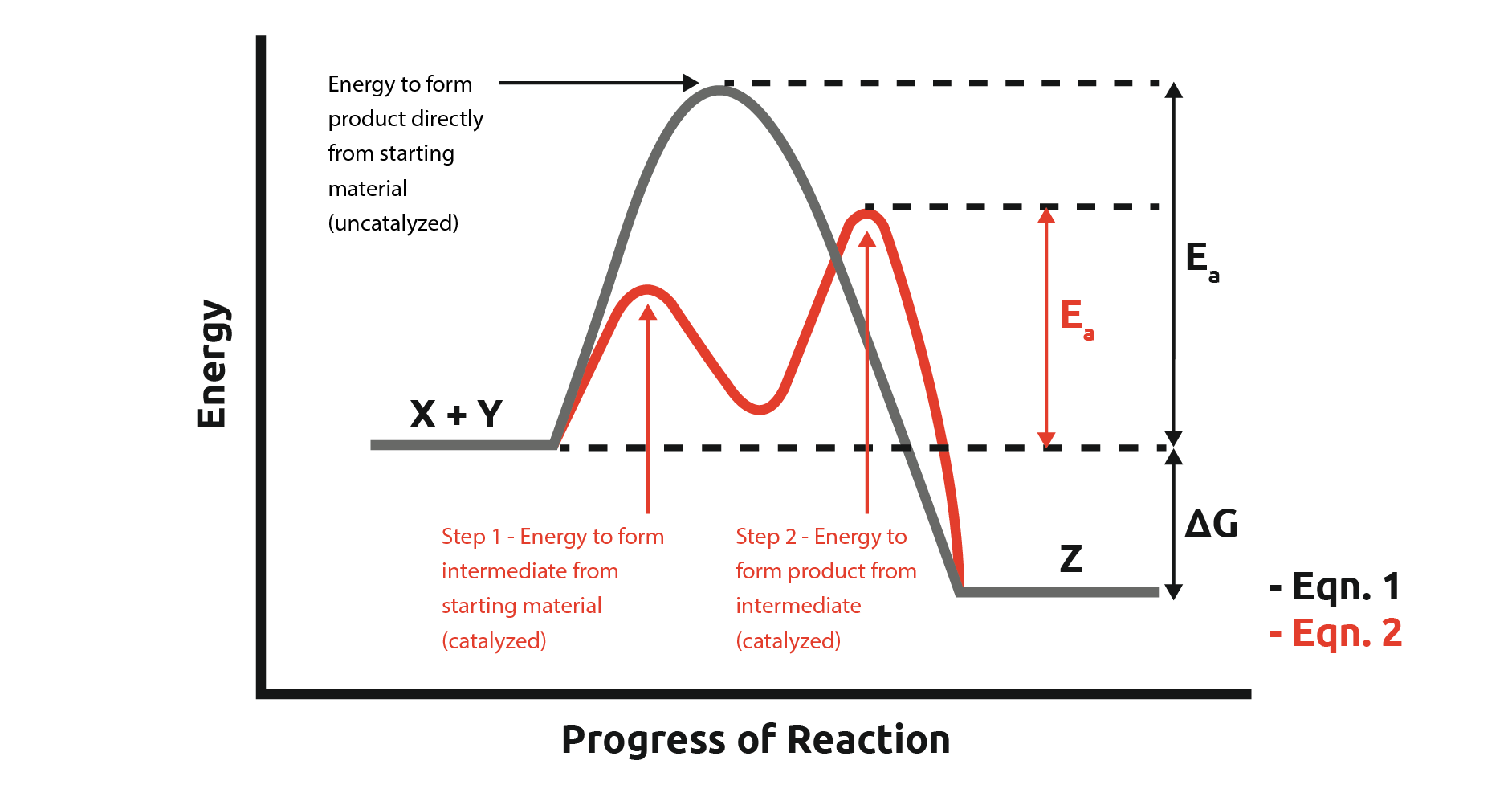
Catalysis In Chemistry . Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. Catalysis is the process of speeding up a reaction using a catalyst. This tutorial review explains some fundamental principles of catalysis and how the mechanisms are studied. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. A catalyst, therefore, does not appear in the overall stoichiometry of the reaction it catalyzes, but it must appear in at least one of the elementary reactions in the mechanism for the catalyzed reaction. Enzymes are naturally occurring catalysts responsible for many. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie.
from www.labunlimited.com
Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. Catalysis is the process of speeding up a reaction using a catalyst. Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. Enzymes are naturally occurring catalysts responsible for many. A catalyst, therefore, does not appear in the overall stoichiometry of the reaction it catalyzes, but it must appear in at least one of the elementary reactions in the mechanism for the catalyzed reaction. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. This tutorial review explains some fundamental principles of catalysis and how the mechanisms are studied. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed.
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited
Catalysis In Chemistry The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. This tutorial review explains some fundamental principles of catalysis and how the mechanisms are studied. Enzymes are naturally occurring catalysts responsible for many. Catalysis is the process of speeding up a reaction using a catalyst. Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. A catalyst, therefore, does not appear in the overall stoichiometry of the reaction it catalyzes, but it must appear in at least one of the elementary reactions in the mechanism for the catalyzed reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work.
From chemistry-europe.onlinelibrary.wiley.com
Dual Catalysis in Organic Synthesis Current Challenges and New Trends Catalysis In Chemistry Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. A catalyst, therefore, does not appear in the overall stoichiometry of the reaction it catalyzes, but it must appear in at least one of the elementary reactions in the mechanism for the catalyzed reaction. Enzymes are naturally. Catalysis In Chemistry.
From isonlab.wordpress.ncsu.edu
Catalysis/Green Chemistry Ison Research Group Catalysis In Chemistry This tutorial review explains some fundamental principles of catalysis and how the mechanisms are studied. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. The word “catalyst” comes from. Catalysis In Chemistry.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalysis In Chemistry Enzymes are naturally occurring catalysts responsible for many. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is the process of speeding up a reaction using a catalyst. This tutorial review explains some fundamental principles of catalysis and how the mechanisms are studied. Catalysts are substances that speed up a reaction but. Catalysis In Chemistry.
From news.virginia.edu
University of Virginia Researchers Uncover New Catalysis Site UVA Today Catalysis In Chemistry The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysis is the process of speeding up a reaction using a catalyst. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. This tutorial review explains some fundamental principles of catalysis. Catalysis In Chemistry.
From www.slideserve.com
PPT 23.5 Features of homogeneous catalysis PowerPoint Presentation Catalysis In Chemistry Catalysis is the process of speeding up a reaction using a catalyst. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalyst, in chemistry, any substance that increases the rate of. Catalysis In Chemistry.
From www.studypool.com
SOLUTION Different types of catalysis in chemistry Studypool Catalysis In Chemistry Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst, therefore, does not appear in the overall stoichiometry of the reaction it catalyzes, but it must appear in at least one of the elementary reactions in the mechanism for the catalyzed reaction. Catalysts are substances that increase the reaction rate of a. Catalysis In Chemistry.
From www.pinterest.com
Catalyst Easy Science in 2022 Energy activities, Chemical reactions Catalysis In Chemistry This tutorial review explains some fundamental principles of catalysis and how the mechanisms are studied. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. A catalyst, therefore, does not appear in the overall stoichiometry of the reaction it catalyzes, but it must appear in at least. Catalysis In Chemistry.
From psu.pb.unizin.org
12.7 Catalysis Chemistry 112 Chapters 1217 of OpenStax General Catalysis In Chemistry Enzymes are naturally occurring catalysts responsible for many. This tutorial review explains some fundamental principles of catalysis and how the mechanisms are studied. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalyst, in chemistry, any substance that increases the rate of a reaction without. Catalysis In Chemistry.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID1803655 Catalysis In Chemistry Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts are substances that increase the reaction rate of a chemical reaction without. Catalysis In Chemistry.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalysis In Chemistry The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. This page looks at the the. Catalysis In Chemistry.
From www.mdpi.com
Molecules Free FullText Is Micellar Catalysis Green Chemistry? Catalysis In Chemistry Catalysis is the process of speeding up a reaction using a catalyst. Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many. This tutorial review explains some fundamental. Catalysis In Chemistry.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Catalysis In Chemistry This tutorial review explains some fundamental principles of catalysis and how the mechanisms are studied. Catalysis is the process of speeding up a reaction using a catalyst. A catalyst, therefore, does not appear in the overall stoichiometry of the reaction it catalyzes, but it must appear in at least one of the elementary reactions in the mechanism for the catalyzed. Catalysis In Chemistry.
From www.science.org
Merging Photoredox Catalysis with Organocatalysis The Direct Catalysis In Chemistry The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysis is the process of speeding up a reaction using a catalyst. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. Enzymes are naturally occurring catalysts responsible for many. Catalysts are. Catalysis In Chemistry.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Catalysis In Chemistry Enzymes are naturally occurring catalysts responsible for many. Catalysis is the process of speeding up a reaction using a catalyst. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. Catalysts are substances that speed up a. Catalysis In Chemistry.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID5829521 Catalysis In Chemistry Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. A catalyst, therefore, does not appear in the overall stoichiometry of the reaction it catalyzes, but it must appear. Catalysis In Chemistry.
From www.britannica.com
Catalysis Chemistry, Classification, & Chemical Reactions Britannica Catalysis In Chemistry Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. This page looks at the the. Catalysis In Chemistry.
From www.eurekalert.org
Catalysis Illustration [IMAGE] EurekAlert! Science News Releases Catalysis In Chemistry Catalysis is the process of speeding up a reaction using a catalyst. Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. Enzymes are naturally occurring catalysts responsible for many. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. This tutorial review explains some fundamental principles. Catalysis In Chemistry.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalysis In Chemistry This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. This tutorial review explains some fundamental principles of catalysis and how the mechanisms are studied. Catalysis is the process of speeding up a reaction using a catalyst. Catalysts are substances that increase the reaction rate of. Catalysis In Chemistry.
From phys.org
New review highlights innovative catalysts Design and application Catalysis In Chemistry Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. Catalysis is the process of speeding up a reaction using a catalyst. This tutorial review explains some fundamental principles of catalysis and how the mechanisms are studied. This page looks at the the different types of catalyst. Catalysis In Chemistry.
From www.researchgate.net
Use of heterogeneous catalysis in various chemistry sectors Download Catalysis In Chemistry Catalysis is the process of speeding up a reaction using a catalyst. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. Catalysts are substances that increase the reaction rate of a. Catalysis In Chemistry.
From pubs.acs.org
Photoredox Catalysis in Organic Chemistry The Journal of Organic Catalysis In Chemistry Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. A catalyst, therefore, does not appear in the overall stoichiometry of. Catalysis In Chemistry.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalysis In Chemistry This tutorial review explains some fundamental principles of catalysis and how the mechanisms are studied. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Enzymes are naturally occurring catalysts responsible for many. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst, therefore, does not appear. Catalysis In Chemistry.
From phys.org
Singleatom catalysis In search of 'holy grails' in catalysis Catalysis In Chemistry A catalyst, therefore, does not appear in the overall stoichiometry of the reaction it catalyzes, but it must appear in at least one of the elementary reactions in the mechanism for the catalyzed reaction. Catalysis is the process of speeding up a reaction using a catalyst. Catalysts are substances that increase the reaction rate of a chemical reaction without being. Catalysis In Chemistry.
From armsingle10.pythonanywhere.com
Beautiful Work Catalyst Chemistry Symbol Class 12 Physics Ncert Notes Catalysis In Chemistry This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. Catalysts are substances that speed up a reaction but which are not consumed by it and do not. Catalysis In Chemistry.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysis In Chemistry This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. The word. Catalysis In Chemistry.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Catalysis In Chemistry This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysis is the process of. Catalysis In Chemistry.
From www.cademix.org
Applications of Heterogeneous Catalysis in Industry Catalysis In Chemistry Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. Enzymes are naturally occurring catalysts responsible for many. A catalyst, therefore, does not appear in the overall stoichiometry of the reaction it catalyzes, but it must appear in at least one of the elementary reactions in the. Catalysis In Chemistry.
From scitechdaily.com
Science Made Simple What Are Catalysts? Catalysis In Chemistry The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. A catalyst, therefore, does not appear in the overall stoichiometry of the reaction it catalyzes, but it must appear in at least one of the elementary reactions in the mechanism for the catalyzed reaction. Catalyst, in chemistry, any substance that increases the rate of a. Catalysis In Chemistry.
From feelbooks.in
Industrial Catalysis Chemistry and Mechanism feelbooks.in Catalysis In Chemistry This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. Enzymes are naturally occurring catalysts responsible for many. This tutorial review explains some. Catalysis In Chemistry.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Catalysis In Chemistry A catalyst, therefore, does not appear in the overall stoichiometry of the reaction it catalyzes, but it must appear in at least one of the elementary reactions in the mechanism for the catalyzed reaction. Enzymes are naturally occurring catalysts responsible for many. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts are. Catalysis In Chemistry.
From www.taylorfrancis.com
Green Catalysis, Green Chemistry, and Organic Syntheses for Sustainable Catalysis In Chemistry Catalysis is the process of speeding up a reaction using a catalyst. A catalyst, therefore, does not appear in the overall stoichiometry of the reaction it catalyzes, but it must appear in at least one of the elementary reactions in the mechanism for the catalyzed reaction. Catalysts are substances that increase the reaction rate of a chemical reaction without being. Catalysis In Chemistry.
From www.empik.com
Catalysis in Organic Chemistry Sabatier Paul Książka w Empik Catalysis In Chemistry Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. Catalysis is the process of speeding up a reaction using a catalyst. Enzymes are naturally occurring catalysts responsible for many. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how. Catalysis In Chemistry.
From thechemistrynotes.com
Catalysis Definition, Types, Advantages, Disadvantages Catalysis In Chemistry Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts are substances that increase the reaction rate of a chemical reaction. Catalysis In Chemistry.
From uscibooks.aip.org
Fundamentals of Asymmetric Catalysis University Science Books Catalysis In Chemistry The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysis is the process of speeding up a reaction using a catalyst. A catalyst, therefore, does not appear in the overall stoichiometry of the reaction it catalyzes, but it must appear in at least one of the elementary reactions in the mechanism for the catalyzed. Catalysis In Chemistry.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalysis In Chemistry Catalysis is the process of speeding up a reaction using a catalyst. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. The. Catalysis In Chemistry.