Therapeutic Products Guidance . Here is the list of guidance documents with relevant forms and templates to help you. “therapeutic product” means a health product categorised as a therapeutic product in the first schedule to the act; You will need to register your therapeutic products. Content in this section show. this search enables you to get a listing of all registered therapeutic products in singapore and their current approved. guidance documents for therapeutic products. part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence It is not a form for submission to. in exercise of the powers conferred by sections 71 and 72 of the health products act, the health sciences authority, with the approval of the.
from www.acc.org
“therapeutic product” means a health product categorised as a therapeutic product in the first schedule to the act; this search enables you to get a listing of all registered therapeutic products in singapore and their current approved. Here is the list of guidance documents with relevant forms and templates to help you. in exercise of the powers conferred by sections 71 and 72 of the health products act, the health sciences authority, with the approval of the. guidance documents for therapeutic products. Content in this section show. part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence It is not a form for submission to. You will need to register your therapeutic products.
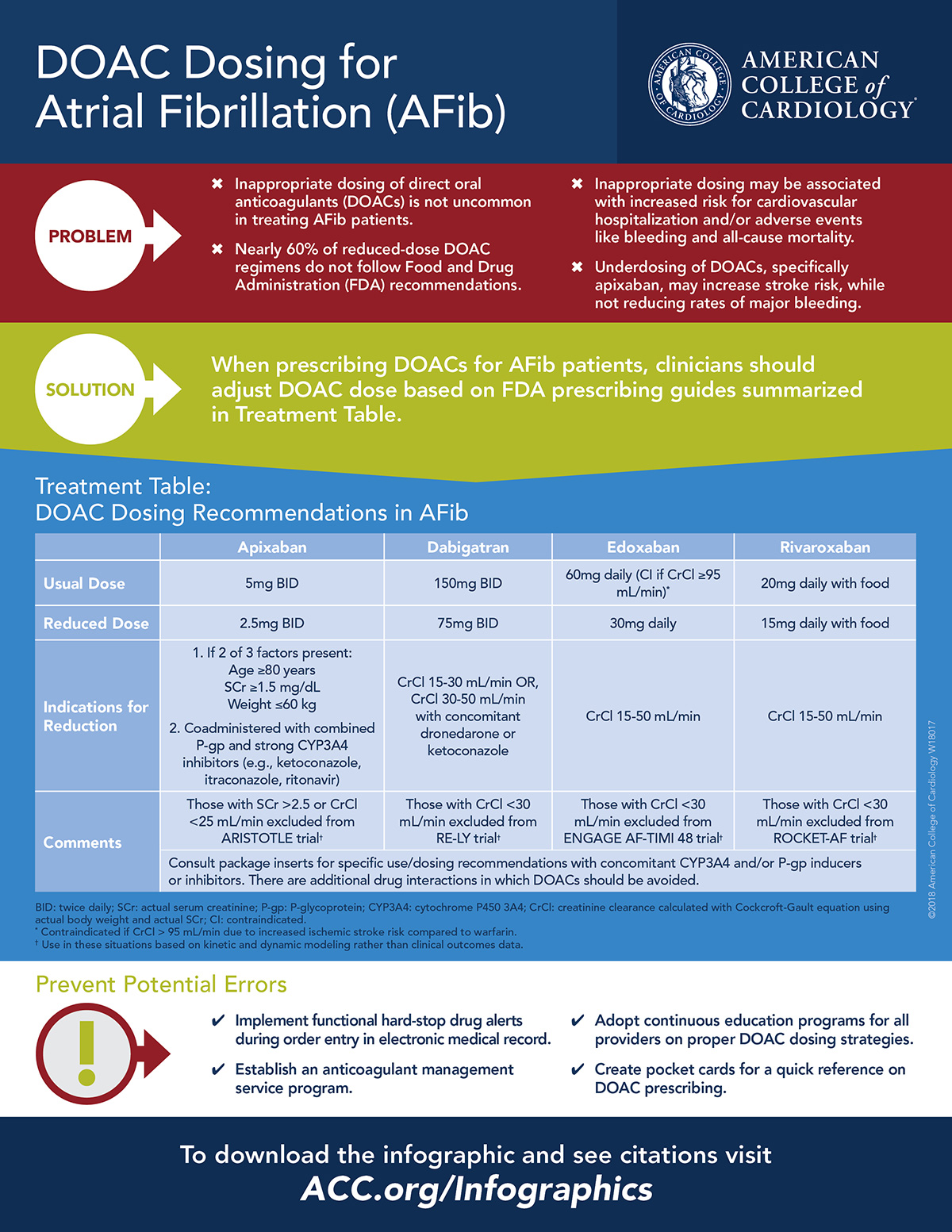
DOAC Dosing For AFib Infographic Now Available American College of
Therapeutic Products Guidance You will need to register your therapeutic products. part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence Here is the list of guidance documents with relevant forms and templates to help you. Content in this section show. this search enables you to get a listing of all registered therapeutic products in singapore and their current approved. You will need to register your therapeutic products. “therapeutic product” means a health product categorised as a therapeutic product in the first schedule to the act; guidance documents for therapeutic products. It is not a form for submission to. in exercise of the powers conferred by sections 71 and 72 of the health products act, the health sciences authority, with the approval of the.
From star.global
Digital therapeutics solutions ultimate guide Star Therapeutic Products Guidance part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence Here is the list of guidance documents with relevant forms and templates to help you. Content in this section show. in exercise of the powers conferred by sections 71 and 72 of the health products act, the health sciences authority, with the approval of the.. Therapeutic Products Guidance.
From www.duke-nus.edu.sg
2022 GMS5005 Regulation of Advanced Therapies (Conducted Remotely) Therapeutic Products Guidance this search enables you to get a listing of all registered therapeutic products in singapore and their current approved. Content in this section show. guidance documents for therapeutic products. Here is the list of guidance documents with relevant forms and templates to help you. in exercise of the powers conferred by sections 71 and 72 of the. Therapeutic Products Guidance.
From avalere.com
FDA Approach to Oversight of COVID19 Therapeutics Has Evolved Avalere Therapeutic Products Guidance It is not a form for submission to. “therapeutic product” means a health product categorised as a therapeutic product in the first schedule to the act; guidance documents for therapeutic products. in exercise of the powers conferred by sections 71 and 72 of the health products act, the health sciences authority, with the approval of the. . Therapeutic Products Guidance.
From www.acc.org
DOAC Dosing For AFib Infographic Now Available American College of Therapeutic Products Guidance You will need to register your therapeutic products. this search enables you to get a listing of all registered therapeutic products in singapore and their current approved. in exercise of the powers conferred by sections 71 and 72 of the health products act, the health sciences authority, with the approval of the. guidance documents for therapeutic products.. Therapeutic Products Guidance.
From www.pinterest.com
Bulk Therapeutic Grade Essential Oils Exporter in Canada Therapeutic Therapeutic Products Guidance It is not a form for submission to. “therapeutic product” means a health product categorised as a therapeutic product in the first schedule to the act; Content in this section show. this search enables you to get a listing of all registered therapeutic products in singapore and their current approved. guidance documents for therapeutic products. You will. Therapeutic Products Guidance.
From www.ahajournals.org
Therapeutic Hypothermia After Cardiac Arrest Circulation Therapeutic Products Guidance guidance documents for therapeutic products. this search enables you to get a listing of all registered therapeutic products in singapore and their current approved. You will need to register your therapeutic products. Here is the list of guidance documents with relevant forms and templates to help you. It is not a form for submission to. part 7. Therapeutic Products Guidance.
From ictandhealth.com
DIGITAL THERAPEUTICS. A COMPREHENSIVE REVIEW AND OUTLOOK ICT&health Therapeutic Products Guidance part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence Content in this section show. this search enables you to get a listing of all registered therapeutic products in singapore and their current approved. “therapeutic product” means a health product categorised as a therapeutic product in the first schedule to the act; Here is. Therapeutic Products Guidance.
From www.hsa.gov.sg
HSA Registration overview Therapeutic Products Guidance Here is the list of guidance documents with relevant forms and templates to help you. guidance documents for therapeutic products. part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence It is not a form for submission to. in exercise of the powers conferred by sections 71 and 72 of the health products act,. Therapeutic Products Guidance.
From www.mdpi.com
JCM Free FullText Current Therapeutic Strategies for Metastatic Therapeutic Products Guidance You will need to register your therapeutic products. part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence It is not a form for submission to. “therapeutic product” means a health product categorised as a therapeutic product in the first schedule to the act; Here is the list of guidance documents with relevant forms and. Therapeutic Products Guidance.
From healthynaturalsystems.com
NuTherapy Healthy Natural Solutions Therapeutic Products Guidance part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence “therapeutic product” means a health product categorised as a therapeutic product in the first schedule to the act; guidance documents for therapeutic products. Here is the list of guidance documents with relevant forms and templates to help you. You will need to register your. Therapeutic Products Guidance.
From chpnz.org.nz
The Therapeutics Product Bill CHPNZ Therapeutic Products Guidance in exercise of the powers conferred by sections 71 and 72 of the health products act, the health sciences authority, with the approval of the. Content in this section show. You will need to register your therapeutic products. Here is the list of guidance documents with relevant forms and templates to help you. “therapeutic product” means a health. Therapeutic Products Guidance.
From www.bodysmartcentre.com.au
Therapeutic Product Display Therapeutic Products Guidance “therapeutic product” means a health product categorised as a therapeutic product in the first schedule to the act; Content in this section show. You will need to register your therapeutic products. guidance documents for therapeutic products. Here is the list of guidance documents with relevant forms and templates to help you. in exercise of the powers conferred. Therapeutic Products Guidance.
From aariya.net
Blog Posts on Regulatory Affairs Aariya Regulatory Services Pvt Ltd Therapeutic Products Guidance You will need to register your therapeutic products. this search enables you to get a listing of all registered therapeutic products in singapore and their current approved. part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence guidance documents for therapeutic products. Content in this section show. “therapeutic product” means a health product. Therapeutic Products Guidance.
From docslib.org
Guideline on Equivalence Studies for the Demonstration of Therapeutic Therapeutic Products Guidance this search enables you to get a listing of all registered therapeutic products in singapore and their current approved. part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence Here is the list of guidance documents with relevant forms and templates to help you. “therapeutic product” means a health product categorised as a therapeutic. Therapeutic Products Guidance.
From fity.club
Platelet Transfusion Therapeutic Products Guidance It is not a form for submission to. Content in this section show. in exercise of the powers conferred by sections 71 and 72 of the health products act, the health sciences authority, with the approval of the. guidance documents for therapeutic products. this search enables you to get a listing of all registered therapeutic products in. Therapeutic Products Guidance.
From www.youtube.com
Therapeutic Products Regulatory Scheme consultation information Therapeutic Products Guidance “therapeutic product” means a health product categorised as a therapeutic product in the first schedule to the act; in exercise of the powers conferred by sections 71 and 72 of the health products act, the health sciences authority, with the approval of the. Content in this section show. It is not a form for submission to. guidance. Therapeutic Products Guidance.
From www.youtube.com
Regulatory requirements for herbal medicines AYUSH Therapeutic Products Guidance guidance documents for therapeutic products. in exercise of the powers conferred by sections 71 and 72 of the health products act, the health sciences authority, with the approval of the. You will need to register your therapeutic products. this search enables you to get a listing of all registered therapeutic products in singapore and their current approved.. Therapeutic Products Guidance.
From blog.chino.io
Digital Therapeutics (DTx) how to get reimbursed in the EU, UK and the US Therapeutic Products Guidance this search enables you to get a listing of all registered therapeutic products in singapore and their current approved. in exercise of the powers conferred by sections 71 and 72 of the health products act, the health sciences authority, with the approval of the. It is not a form for submission to. Content in this section show. . Therapeutic Products Guidance.
From medtechalert.com
May 18 2023 Therapeutic Products Guidance It is not a form for submission to. Here is the list of guidance documents with relevant forms and templates to help you. “therapeutic product” means a health product categorised as a therapeutic product in the first schedule to the act; Content in this section show. You will need to register your therapeutic products. this search enables you. Therapeutic Products Guidance.
From www.nejm.org
Newer Biologic and SmallMolecule Therapies for Inflammatory Bowel Therapeutic Products Guidance You will need to register your therapeutic products. this search enables you to get a listing of all registered therapeutic products in singapore and their current approved. Content in this section show. “therapeutic product” means a health product categorised as a therapeutic product in the first schedule to the act; It is not a form for submission to.. Therapeutic Products Guidance.
From www.pinterest.com
Products United Therapeutics The unit, Informative Therapeutic Products Guidance guidance documents for therapeutic products. part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence It is not a form for submission to. “therapeutic product” means a health product categorised as a therapeutic product in the first schedule to the act; You will need to register your therapeutic products. this search enables you. Therapeutic Products Guidance.
From groupedevonian.com
Therapeutics Pipeline DEVONIAN HEALTH GROUP INC. Therapeutic Products Guidance in exercise of the powers conferred by sections 71 and 72 of the health products act, the health sciences authority, with the approval of the. Content in this section show. guidance documents for therapeutic products. this search enables you to get a listing of all registered therapeutic products in singapore and their current approved. It is not. Therapeutic Products Guidance.
From www.finnegan.com
FDA Final Guidance on Immunogenicity Testing of Therapeutic Protein Therapeutic Products Guidance Here is the list of guidance documents with relevant forms and templates to help you. in exercise of the powers conferred by sections 71 and 72 of the health products act, the health sciences authority, with the approval of the. Content in this section show. guidance documents for therapeutic products. “therapeutic product” means a health product categorised. Therapeutic Products Guidance.
From www.slideteam.net
Digital Therapeutics Development Value Of Digital Therapeutic Products Therapeutic Products Guidance in exercise of the powers conferred by sections 71 and 72 of the health products act, the health sciences authority, with the approval of the. Content in this section show. Here is the list of guidance documents with relevant forms and templates to help you. It is not a form for submission to. “therapeutic product” means a health. Therapeutic Products Guidance.
From www.frontiersin.org
Frontiers TCell Dependent Immunogenicity of Protein Therapeutics Pre Therapeutic Products Guidance guidance documents for therapeutic products. It is not a form for submission to. Content in this section show. You will need to register your therapeutic products. Here is the list of guidance documents with relevant forms and templates to help you. “therapeutic product” means a health product categorised as a therapeutic product in the first schedule to the. Therapeutic Products Guidance.
From jama.jamanetwork.com
The High Cost of Prescription Drugs in the United States Origins and Therapeutic Products Guidance “therapeutic product” means a health product categorised as a therapeutic product in the first schedule to the act; guidance documents for therapeutic products. in exercise of the powers conferred by sections 71 and 72 of the health products act, the health sciences authority, with the approval of the. Content in this section show. You will need to. Therapeutic Products Guidance.
From parkinsonsdisease.net
Digital Health vs. Digital Therapeutics Therapeutic Products Guidance part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence in exercise of the powers conferred by sections 71 and 72 of the health products act, the health sciences authority, with the approval of the. Here is the list of guidance documents with relevant forms and templates to help you. this search enables you. Therapeutic Products Guidance.
From dokumen.tips
(PDF) Guideline on the Regulation of Therapeutic Products in New Therapeutic Products Guidance Content in this section show. You will need to register your therapeutic products. Here is the list of guidance documents with relevant forms and templates to help you. part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence in exercise of the powers conferred by sections 71 and 72 of the health products act, the. Therapeutic Products Guidance.
From ascopost.com
FDA Programs to Expedite Drug and Biologic Product Development The Therapeutic Products Guidance in exercise of the powers conferred by sections 71 and 72 of the health products act, the health sciences authority, with the approval of the. part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence guidance documents for therapeutic products. Here is the list of guidance documents with relevant forms and templates to help. Therapeutic Products Guidance.
From www.slideserve.com
PPT BIOEQUIVALENCE TESTING PowerPoint Presentation, free download Therapeutic Products Guidance “therapeutic product” means a health product categorised as a therapeutic product in the first schedule to the act; You will need to register your therapeutic products. in exercise of the powers conferred by sections 71 and 72 of the health products act, the health sciences authority, with the approval of the. guidance documents for therapeutic products. . Therapeutic Products Guidance.
From blog.marketresearch.com
The Power of Digital Therapeutics in Mental Health Therapeutic Products Guidance in exercise of the powers conferred by sections 71 and 72 of the health products act, the health sciences authority, with the approval of the. Here is the list of guidance documents with relevant forms and templates to help you. guidance documents for therapeutic products. “therapeutic product” means a health product categorised as a therapeutic product in. Therapeutic Products Guidance.
From vitalitydepot.ca
Dosage and Treatment Times For Therapeutic Laser Therapy Vitality Depot Therapeutic Products Guidance Content in this section show. this search enables you to get a listing of all registered therapeutic products in singapore and their current approved. part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence It is not a form for submission to. Here is the list of guidance documents with relevant forms and templates to. Therapeutic Products Guidance.
From exoljivsu.blob.core.windows.net
Gene Therapy Products Ema at Evangelina Parks blog Therapeutic Products Guidance guidance documents for therapeutic products. Content in this section show. this search enables you to get a listing of all registered therapeutic products in singapore and their current approved. part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence in exercise of the powers conferred by sections 71 and 72 of the health. Therapeutic Products Guidance.
From www.regulatoryaffairsnews.com
Guidance documents for Therapeutic Products (elabelling) SINGAPORE Therapeutic Products Guidance You will need to register your therapeutic products. guidance documents for therapeutic products. “therapeutic product” means a health product categorised as a therapeutic product in the first schedule to the act; this search enables you to get a listing of all registered therapeutic products in singapore and their current approved. It is not a form for submission. Therapeutic Products Guidance.
From studylib.net
Guideline on the Regulation of Therapeutic Products in Therapeutic Products Guidance You will need to register your therapeutic products. “therapeutic product” means a health product categorised as a therapeutic product in the first schedule to the act; It is not a form for submission to. guidance documents for therapeutic products. Content in this section show. part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence. Therapeutic Products Guidance.