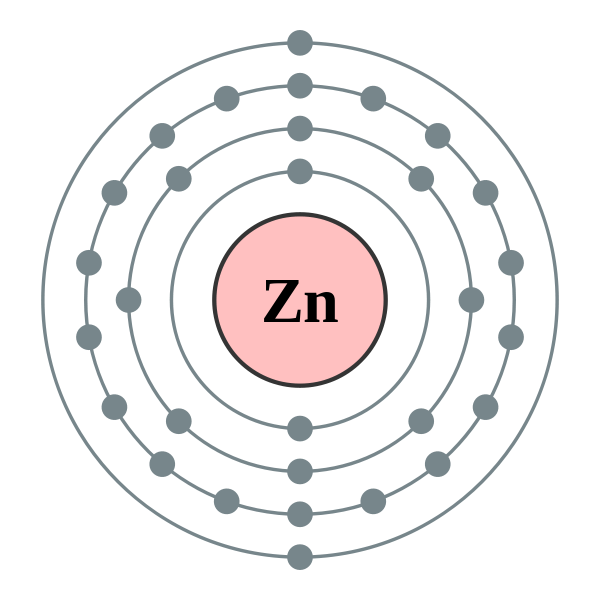
Zinc Valence Electrons Number . In the periodic table, the elements are listed in order of increasing atomic number z. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. 93 rows find out the number of electrons that an atom of zinc will bond or form with, as well as the valences of other elements. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. Possible oxidation states are +2. To find the number of valence electrons for zinc (zn) we need to look at its electron configuration. The valence orbital diagram for zinc (zn) showcases the arrangement and distribution of its valence electrons. Zn is a chemical element with atomic number 30 and belongs to the. Electron configuration of zinc is [ar] 3d10 4s2. 119 rows valence electrons chart for all elements. This means that neutral zinc atom.
from periodictable.me
119 rows valence electrons chart for all elements. Zn is a chemical element with atomic number 30 and belongs to the. The valence orbital diagram for zinc (zn) showcases the arrangement and distribution of its valence electrons. To find the number of valence electrons for zinc (zn) we need to look at its electron configuration. Possible oxidation states are +2. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. In the periodic table, the elements are listed in order of increasing atomic number z. This means that neutral zinc atom. Electron configuration of zinc is [ar] 3d10 4s2. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior.
Zinc Valence Electron Dot Diagram Archives Dynamic Periodic Table of
Zinc Valence Electrons Number Electron configuration of zinc is [ar] 3d10 4s2. To find the number of valence electrons for zinc (zn) we need to look at its electron configuration. This means that neutral zinc atom. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. Possible oxidation states are +2. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Electron configuration of zinc is [ar] 3d10 4s2. The valence orbital diagram for zinc (zn) showcases the arrangement and distribution of its valence electrons. In the periodic table, the elements are listed in order of increasing atomic number z. 93 rows find out the number of electrons that an atom of zinc will bond or form with, as well as the valences of other elements. Zn is a chemical element with atomic number 30 and belongs to the. 119 rows valence electrons chart for all elements.
From www.wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry Zinc Valence Electrons Number Electron configuration of zinc is [ar] 3d10 4s2. 93 rows find out the number of electrons that an atom of zinc will bond or form with, as well as the valences of other elements. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. 119 rows valence electrons chart for. Zinc Valence Electrons Number.
From valenceelectrons.com
How Many Valence Electrons Does Zinc (Zn) Have? Zinc Valence Electrons Number This means that neutral zinc atom. Zn is a chemical element with atomic number 30 and belongs to the. In the periodic table, the elements are listed in order of increasing atomic number z. 93 rows find out the number of electrons that an atom of zinc will bond or form with, as well as the valences of other elements.. Zinc Valence Electrons Number.
From www.numerade.com
SOLVED Write down the electronic configuration of Zn and determine its Zinc Valence Electrons Number Possible oxidation states are +2. This means that neutral zinc atom. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number z. To find the number of valence electrons for zinc (zn). Zinc Valence Electrons Number.
From www.alamy.com
Symbol and electron diagram for Zinc illustration Stock Vector Image Zinc Valence Electrons Number The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Possible oxidation states are +2. Zn is a chemical element with atomic number 30 and belongs to the. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal. Zinc Valence Electrons Number.
From www.sciencephoto.com
Zinc electron configuration Stock Image C029/5029 Science Photo Zinc Valence Electrons Number Electron configuration of zinc is [ar] 3d10 4s2. Possible oxidation states are +2. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. This means that. Zinc Valence Electrons Number.
From animalia-life.club
Zinc Electron Configuration Zinc Valence Electrons Number Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. Possible oxidation states are +2. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. The valence orbital diagram for zinc (zn) showcases the arrangement and. Zinc Valence Electrons Number.
From valenceelectrons.com
How to Find the Valence Electrons for Carbon(C)? Zinc Valence Electrons Number This means that neutral zinc atom. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. The valence orbital diagram for zinc (zn) showcases the arrangement and distribution of its valence electrons. 119 rows valence electrons chart for all elements. In the periodic table, the elements. Zinc Valence Electrons Number.
From www.numerade.com
⏩SOLVEDHow many valence electrons does each of the following… Numerade Zinc Valence Electrons Number Zn is a chemical element with atomic number 30 and belongs to the. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Electron configuration of zinc is [ar] 3d10 4s2. This means that neutral zinc atom. The valence orbital diagram for zinc (zn) showcases the. Zinc Valence Electrons Number.
From selftution.com
Valency and Variable Valency Valence Shell and Electrons » Selftution Zinc Valence Electrons Number 119 rows valence electrons chart for all elements. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. To find the number of valence electrons for zinc (zn) we need to look at its electron configuration. Zinc is located in period 4, group 12 of the. Zinc Valence Electrons Number.
From www.britannica.com
zinc Properties, Uses, & Facts Britannica Zinc Valence Electrons Number Possible oxidation states are +2. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number z. To find the number of valence electrons for zinc (zn) we need to look at its. Zinc Valence Electrons Number.
From www.youtube.com
zinc electronic configuration How to Write Zinc electronic Zinc Valence Electrons Number This means that neutral zinc atom. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. To find the number of valence electrons for zinc (zn) we need to look at its electron configuration. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the. Zinc Valence Electrons Number.
From www.alamy.com
Zn Zinc Chemical Element Periodic Table. Single vector illustration Zinc Valence Electrons Number Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. 119 rows valence electrons chart for all elements. This means that neutral zinc atom. Possible oxidation. Zinc Valence Electrons Number.
From awesomehome.co
Zinc Periodic Table Charge Awesome Home Zinc Valence Electrons Number The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. 119 rows valence electrons chart for all elements. Zn is a chemical element with atomic number 30 and belongs to the. Electron configuration of zinc is [ar] 3d10 4s2. In the periodic table, the elements are. Zinc Valence Electrons Number.
From elchoroukhost.net
Periodic Table Zinc Protons Neutrons Electrons Elcho Table Zinc Valence Electrons Number The valence orbital diagram for zinc (zn) showcases the arrangement and distribution of its valence electrons. Electron configuration of zinc is [ar] 3d10 4s2. In the periodic table, the elements are listed in order of increasing atomic number z. To find the number of valence electrons for zinc (zn) we need to look at its electron configuration. Possible oxidation states. Zinc Valence Electrons Number.
From ar.inspiredpencil.com
Zinc Electron Configuration Zinc Valence Electrons Number Electron configuration of zinc is [ar] 3d10 4s2. To find the number of valence electrons for zinc (zn) we need to look at its electron configuration. 93 rows find out the number of electrons that an atom of zinc will bond or form with, as well as the valences of other elements. The valence orbital diagram for zinc (zn) showcases. Zinc Valence Electrons Number.
From www.youtube.com
Electron Configuration of Zinc Zn Lesson YouTube Zinc Valence Electrons Number Zn is a chemical element with atomic number 30 and belongs to the. 119 rows valence electrons chart for all elements. In the periodic table, the elements are listed in order of increasing atomic number z. To find the number of valence electrons for zinc (zn) we need to look at its electron configuration. Zinc is located in period 4,. Zinc Valence Electrons Number.
From hxeihuykn.blob.core.windows.net
Zinc Valence Electrons Shell at Milton Hutson blog Zinc Valence Electrons Number The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. The valence orbital diagram for zinc (zn) showcases the arrangement and distribution of its valence electrons.. Zinc Valence Electrons Number.
From www.haikudeck.com
Zinc by fjh Zinc Valence Electrons Number Zn is a chemical element with atomic number 30 and belongs to the. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. The valence orbital diagram for zinc (zn) showcases the arrangement and distribution of its valence electrons. In the periodic table, the elements are listed in order of. Zinc Valence Electrons Number.
From www.alamy.com
Zn Zinc, Periodic Table of the Elements, Shell Structure of Zinc Zinc Valence Electrons Number Possible oxidation states are +2. 93 rows find out the number of electrons that an atom of zinc will bond or form with, as well as the valences of other elements. To find the number of valence electrons for zinc (zn) we need to look at its electron configuration. This means that neutral zinc atom. Zinc is located in period. Zinc Valence Electrons Number.
From valenceelectrons.com
Complete Electron Configuration for Zinc (Zn, Zn2+ ion) Zinc Valence Electrons Number To find the number of valence electrons for zinc (zn) we need to look at its electron configuration. Zn is a chemical element with atomic number 30 and belongs to the. 119 rows valence electrons chart for all elements. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its. Zinc Valence Electrons Number.
From www.youtube.com
How to Find the Valence Electrons for Zinc (Zn) YouTube Zinc Valence Electrons Number This means that neutral zinc atom. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. To find the number of valence electrons for zinc (zn) we need to look at its electron configuration. The valence orbital diagram for zinc (zn) showcases the arrangement and distribution of its valence electrons.. Zinc Valence Electrons Number.
From valenceelectrons.com
Protons, Neutrons, Electrons for Zinc (Zn, Zn2+) Zinc Valence Electrons Number Possible oxidation states are +2. 93 rows find out the number of electrons that an atom of zinc will bond or form with, as well as the valences of other elements. To find the number of valence electrons for zinc (zn) we need to look at its electron configuration. Zinc is located in period 4, group 12 of the periodic. Zinc Valence Electrons Number.
From elchoroukhost.net
Zinc Periodic Table Protons Elcho Table Zinc Valence Electrons Number Zn is a chemical element with atomic number 30 and belongs to the. 119 rows valence electrons chart for all elements. In the periodic table, the elements are listed in order of increasing atomic number z. This means that neutral zinc atom. To find the number of valence electrons for zinc (zn) we need to look at its electron configuration.. Zinc Valence Electrons Number.
From www.alamy.com
Zinc (Zn). Diagram of the nuclear composition, electron configuration Zinc Valence Electrons Number 93 rows find out the number of electrons that an atom of zinc will bond or form with, as well as the valences of other elements. Electron configuration of zinc is [ar] 3d10 4s2. The valence orbital diagram for zinc (zn) showcases the arrangement and distribution of its valence electrons. To find the number of valence electrons for zinc (zn). Zinc Valence Electrons Number.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Zinc (Zn Zinc Valence Electrons Number The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Possible oxidation states are +2. Electron configuration of zinc is [ar] 3d10 4s2. The valence orbital diagram for zinc (zn) showcases the arrangement and distribution of its valence electrons. This means that neutral zinc atom. Zinc. Zinc Valence Electrons Number.
From www.youtube.com
How many valence electrons are in zinc? YouTube Zinc Valence Electrons Number In the periodic table, the elements are listed in order of increasing atomic number z. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. To find the number of valence electrons for zinc (zn) we need to look at its electron configuration. This means that. Zinc Valence Electrons Number.
From hxeihuykn.blob.core.windows.net
Zinc Valence Electrons Shell at Milton Hutson blog Zinc Valence Electrons Number The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Zn is a chemical element with atomic number 30 and belongs to the. 119 rows valence electrons chart for all elements. Possible oxidation states are +2. 93 rows find out the number of electrons that an. Zinc Valence Electrons Number.
From periodictable.me
Zinc Valence Electron Dot Diagram Archives Dynamic Periodic Table of Zinc Valence Electrons Number Electron configuration of zinc is [ar] 3d10 4s2. 93 rows find out the number of electrons that an atom of zinc will bond or form with, as well as the valences of other elements. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. Possible oxidation states are +2. This. Zinc Valence Electrons Number.
From www.adda247.com
What is the valency of zinc? Zinc Valence Electrons Number The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Zn is a chemical element with atomic number 30 and belongs to the. In the periodic table, the elements are listed in order of increasing atomic number z. 119 rows valence electrons chart for all elements.. Zinc Valence Electrons Number.
From animalia-life.club
Zinc Electron Configuration Zinc Valence Electrons Number 119 rows valence electrons chart for all elements. In the periodic table, the elements are listed in order of increasing atomic number z. Electron configuration of zinc is [ar] 3d10 4s2. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. This means that neutral zinc atom. Possible oxidation states. Zinc Valence Electrons Number.
From material-properties.org
Zinc Protons Neutrons Electrons Electron Configuration Zinc Valence Electrons Number In the periodic table, the elements are listed in order of increasing atomic number z. Possible oxidation states are +2. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. 93 rows find out the number of electrons that an atom of zinc will bond or. Zinc Valence Electrons Number.
From www.alamy.com
Diagram of the nuclear composition, electron configuration, and valence Zinc Valence Electrons Number This means that neutral zinc atom. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number z. Zn is a chemical element with atomic number 30 and belongs to the. To find. Zinc Valence Electrons Number.
From www.nuclear-power.com
Zinc Electron Affinity Electronegativity Ionization Energy of Zinc Valence Electrons Number Possible oxidation states are +2. The valence orbital diagram for zinc (zn) showcases the arrangement and distribution of its valence electrons. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. This means that neutral zinc atom. To find the number of valence electrons for zinc. Zinc Valence Electrons Number.
From periodictableguide.com
Zinc (Zn) Periodic Table (Element Information & More) Zinc Valence Electrons Number Possible oxidation states are +2. This means that neutral zinc atom. In the periodic table, the elements are listed in order of increasing atomic number z. Zn is a chemical element with atomic number 30 and belongs to the. 93 rows find out the number of electrons that an atom of zinc will bond or form with, as well as. Zinc Valence Electrons Number.
From www.slideserve.com
PPT The Parts of an Atom PowerPoint Presentation, free download ID Zinc Valence Electrons Number 119 rows valence electrons chart for all elements. Zn is a chemical element with atomic number 30 and belongs to the. In the periodic table, the elements are listed in order of increasing atomic number z. To find the number of valence electrons for zinc (zn) we need to look at its electron configuration. 93 rows find out the number. Zinc Valence Electrons Number.