Barometric Formula Explanation . The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the. The barometric formula describes the relationship between the pressure of the atmosphere and the altitude above sea level. If we let \(\eta\) be the number of molecules per unit volume, \(\eta. It is used to model. Either of the latter relationships is frequently called the barometric formula. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! In contrast to incompressible fluids, the density of gases changes as a function of the applied pressure. If the pressure is given in millimeters of mercury \(\left( \text{mmhg} \right),\) the barometric formula is written in the form:
from www.tec-science.com
The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the. It is used to model. Either of the latter relationships is frequently called the barometric formula. If the pressure is given in millimeters of mercury \(\left( \text{mmhg} \right),\) the barometric formula is written in the form: The barometric formula describes the relationship between the pressure of the atmosphere and the altitude above sea level. If we let \(\eta\) be the number of molecules per unit volume, \(\eta. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! In contrast to incompressible fluids, the density of gases changes as a function of the applied pressure.
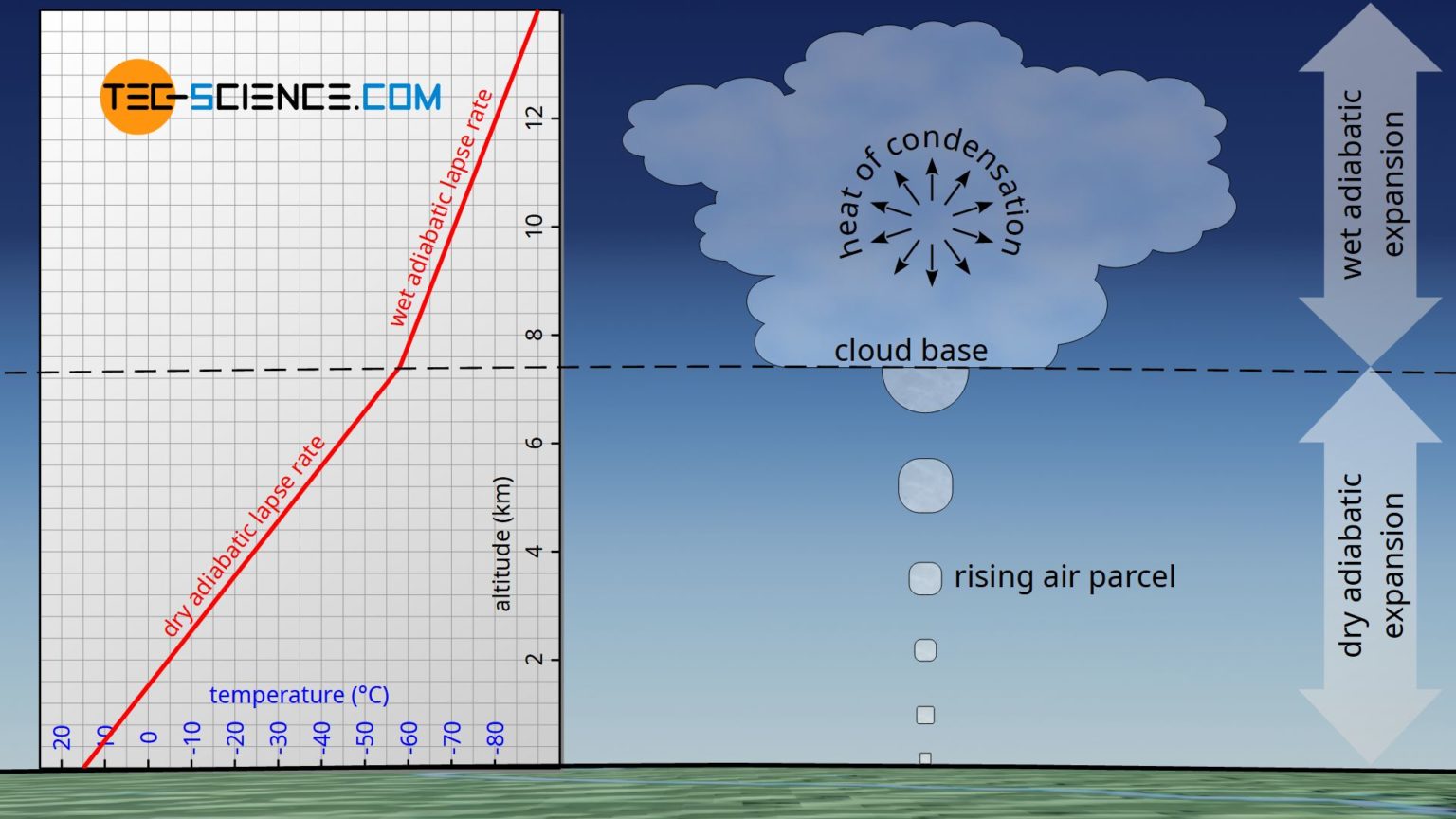
Derivation of the barometric formula (adiabatic atmosphere) tecscience
Barometric Formula Explanation In contrast to incompressible fluids, the density of gases changes as a function of the applied pressure. If we let \(\eta\) be the number of molecules per unit volume, \(\eta. In contrast to incompressible fluids, the density of gases changes as a function of the applied pressure. Either of the latter relationships is frequently called the barometric formula. The barometric formula describes the relationship between the pressure of the atmosphere and the altitude above sea level. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the. If the pressure is given in millimeters of mercury \(\left( \text{mmhg} \right),\) the barometric formula is written in the form: It is used to model.
From www.gkseries.com
Atmospheric Pressure Geography Barometric Formula Explanation The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! The barometric formula describes the relationship between the pressure of the atmosphere and the altitude above sea level. If the pressure is given in millimeters of mercury \(\left( \text{mmhg} \right),\) the barometric formula is written in. Barometric Formula Explanation.
From es.scribd.com
The Barometric Formula V2 PDF Gases Presión atmosférica Barometric Formula Explanation The barometric formula describes the relationship between the pressure of the atmosphere and the altitude above sea level. In contrast to incompressible fluids, the density of gases changes as a function of the applied pressure. Either of the latter relationships is frequently called the barometric formula. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular. Barometric Formula Explanation.
From www.tec-science.com
Derivation of the barometric formula (adiabatic atmosphere) tecscience Barometric Formula Explanation If the pressure is given in millimeters of mercury \(\left( \text{mmhg} \right),\) the barometric formula is written in the form: The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! If we let \(\eta\) be the number of molecules per unit volume, \(\eta. The barometric formula. Barometric Formula Explanation.
From www.youtube.com
How to calculate barometric altitude YouTube Barometric Formula Explanation The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the. The barometric formula describes the relationship between the pressure of the atmosphere and the altitude above sea level. It is used to model. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of. Barometric Formula Explanation.
From www.slideserve.com
PPT Lecture 5 Barometric formula and the Boltzmann equation Barometric Formula Explanation If we let \(\eta\) be the number of molecules per unit volume, \(\eta. In contrast to incompressible fluids, the density of gases changes as a function of the applied pressure. If the pressure is given in millimeters of mercury \(\left( \text{mmhg} \right),\) the barometric formula is written in the form: The barometric formula describes the relationship between the pressure of. Barometric Formula Explanation.
From www.youtube.com
Deriving the Barometric Formula for Pressure YouTube Barometric Formula Explanation It is used to model. The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the. If we let \(\eta\) be the number of molecules per unit volume, \(\eta. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some. Barometric Formula Explanation.
From www.wikihow.com
How to Calculate Barometric Pressure 6 Steps (with Pictures) Barometric Formula Explanation If we let \(\eta\) be the number of molecules per unit volume, \(\eta. The barometric formula describes the relationship between the pressure of the atmosphere and the altitude above sea level. Either of the latter relationships is frequently called the barometric formula. It is used to model. If the pressure is given in millimeters of mercury \(\left( \text{mmhg} \right),\) the. Barometric Formula Explanation.
From www.slideserve.com
PPT Lecture 5 Barometric formula and the Boltzmann equation Barometric Formula Explanation If the pressure is given in millimeters of mercury \(\left( \text{mmhg} \right),\) the barometric formula is written in the form: In contrast to incompressible fluids, the density of gases changes as a function of the applied pressure. If we let \(\eta\) be the number of molecules per unit volume, \(\eta. Either of the latter relationships is frequently called the barometric. Barometric Formula Explanation.
From www.slideserve.com
PPT Barometric Pressure PowerPoint Presentation, free download ID Barometric Formula Explanation The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! If we let \(\eta\) be the number of molecules per unit volume, \(\eta. The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and. Barometric Formula Explanation.
From www.studypool.com
SOLUTION Derivation of Barometric Equation Studypool Barometric Formula Explanation Either of the latter relationships is frequently called the barometric formula. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! In contrast to incompressible fluids, the density of gases changes as a function of the applied pressure. If the pressure is given in millimeters of. Barometric Formula Explanation.
From www.tec-science.com
Derivation of the barometric formula (adiabatic atmosphere) tecscience Barometric Formula Explanation It is used to model. The barometric formula describes the relationship between the pressure of the atmosphere and the altitude above sea level. The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the. If the pressure is given in millimeters of mercury \(\left( \text{mmhg} \right),\) the. Barometric Formula Explanation.
From en.ppt-online.org
Physics 1 for KMA online presentation Barometric Formula Explanation In contrast to incompressible fluids, the density of gases changes as a function of the applied pressure. It is used to model. The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the. If we let \(\eta\) be the number of molecules per unit volume, \(\eta. The. Barometric Formula Explanation.
From es.scribd.com
The Barometric Formula PDF Gases Presión Barometric Formula Explanation Either of the latter relationships is frequently called the barometric formula. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! The barometric formula describes the relationship between the pressure of the atmosphere and the altitude above sea level. If the pressure is given in millimeters. Barometric Formula Explanation.
From www.slideserve.com
PPT Lecture 5 Barometric formula and the Boltzmann equation Barometric Formula Explanation If we let \(\eta\) be the number of molecules per unit volume, \(\eta. The barometric formula describes the relationship between the pressure of the atmosphere and the altitude above sea level. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! In contrast to incompressible fluids,. Barometric Formula Explanation.
From www.youtube.com
IGCSE Physics (0625) Atmospheric Pressure YouTube Barometric Formula Explanation The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! In contrast to incompressible fluids, the density of gases changes as a. Barometric Formula Explanation.
From www.slideserve.com
PPT Lecture 5 Barometric formula and the Boltzmann equation Barometric Formula Explanation The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the. If the pressure is given in millimeters of mercury \(\left( \text{mmhg} \right),\). Barometric Formula Explanation.
From www.youtube.com
Barometric Formula Part 1 YouTube Barometric Formula Explanation If the pressure is given in millimeters of mercury \(\left( \text{mmhg} \right),\) the barometric formula is written in the form: The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! In contrast to incompressible fluids, the density of gases changes as a function of the applied. Barometric Formula Explanation.
From www.youtube.com
Barometric formula(lecture 1)Professor Aziz Atif YouTube Barometric Formula Explanation Either of the latter relationships is frequently called the barometric formula. If we let \(\eta\) be the number of molecules per unit volume, \(\eta. The barometric formula describes the relationship between the pressure of the atmosphere and the altitude above sea level. If the pressure is given in millimeters of mercury \(\left( \text{mmhg} \right),\) the barometric formula is written in. Barometric Formula Explanation.
From www.youtube.com
Barometric Formula Part 2 YouTube Barometric Formula Explanation The barometric formula describes the relationship between the pressure of the atmosphere and the altitude above sea level. In contrast to incompressible fluids, the density of gases changes as a function of the applied pressure. It is used to model. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z. Barometric Formula Explanation.
From www.youtube.com
Barometric Equation YouTube Barometric Formula Explanation Either of the latter relationships is frequently called the barometric formula. It is used to model. In contrast to incompressible fluids, the density of gases changes as a function of the applied pressure. The barometric formula describes the relationship between the pressure of the atmosphere and the altitude above sea level. If we let \(\eta\) be the number of molecules. Barometric Formula Explanation.
From www.youtube.com
Barometric Equations Variation of Pressure with Altitude. Fluid Barometric Formula Explanation The barometric formula describes the relationship between the pressure of the atmosphere and the altitude above sea level. If we let \(\eta\) be the number of molecules per unit volume, \(\eta. In contrast to incompressible fluids, the density of gases changes as a function of the applied pressure. The development of the barometric formula makes use of a number of. Barometric Formula Explanation.
From modern-physics.org
Barometric Formula Altitude, Pressure & Temperature Barometric Formula Explanation The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! The barometric formula describes the relationship between the pressure of the atmosphere and the altitude above sea level. In contrast to incompressible fluids, the density of gases changes as a function of the applied pressure. It. Barometric Formula Explanation.
From www.youtube.com
The Barometric Formula YouTube Barometric Formula Explanation The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! Either of the latter relationships is frequently called the barometric formula. If the pressure is given in millimeters of mercury \(\left( \text{mmhg} \right),\) the barometric formula is written in the form: The development of the barometric. Barometric Formula Explanation.
From www.chegg.com
Solved 4 The barometric equation (16 points) (a) Consider a Barometric Formula Explanation The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the. Either of the latter relationships is frequently called the barometric formula. It is used to model. The barometric formula describes the relationship between the pressure of the atmosphere and the altitude above sea level. The barometric. Barometric Formula Explanation.
From byjus.com
Explain the how the variation of atmospheric pressure with height is Barometric Formula Explanation In contrast to incompressible fluids, the density of gases changes as a function of the applied pressure. Either of the latter relationships is frequently called the barometric formula. If we let \(\eta\) be the number of molecules per unit volume, \(\eta. If the pressure is given in millimeters of mercury \(\left( \text{mmhg} \right),\) the barometric formula is written in the. Barometric Formula Explanation.
From www.youtube.com
Barometric Equation Derivation. YouTube Barometric Formula Explanation The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! The barometric formula describes the relationship between the pressure of the atmosphere and the altitude above sea level. If the pressure is given in millimeters of mercury \(\left( \text{mmhg} \right),\) the barometric formula is written in. Barometric Formula Explanation.
From www.slideserve.com
PPT Lecture 5 Barometric formula and the Boltzmann equation Barometric Formula Explanation The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! The barometric formula describes the relationship between the pressure of the atmosphere and the altitude above sea level. In contrast to incompressible fluids, the density of gases changes as a function of the applied pressure. The. Barometric Formula Explanation.
From weatherstationary.com
Barometric Pressure Headaches Cause & Treatment Barometric Formula Explanation If the pressure is given in millimeters of mercury \(\left( \text{mmhg} \right),\) the barometric formula is written in the form: The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! The development of the barometric formula makes use of a number of concepts from kinetic theory,. Barometric Formula Explanation.
From www.slideserve.com
PPT Lecture 4 Barometric formula and the Boltzmann equation Simple Barometric Formula Explanation The barometric formula describes the relationship between the pressure of the atmosphere and the altitude above sea level. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! The development of the barometric formula makes use of a number of concepts from kinetic theory, such as. Barometric Formula Explanation.
From www.youtube.com
Derivation of Barometric Formula Barometric Distribution LawUrdu Barometric Formula Explanation It is used to model. In contrast to incompressible fluids, the density of gases changes as a function of the applied pressure. Either of the latter relationships is frequently called the barometric formula. If we let \(\eta\) be the number of molecules per unit volume, \(\eta. The development of the barometric formula makes use of a number of concepts from. Barometric Formula Explanation.
From www.tec-science.com
Derivation of the barometric formula (adiabatic atmosphere) tecscience Barometric Formula Explanation In contrast to incompressible fluids, the density of gases changes as a function of the applied pressure. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! The barometric formula describes the relationship between the pressure of the atmosphere and the altitude above sea level. Either. Barometric Formula Explanation.
From instrumentationtools.com
What is a Barometer? Instrumentation Tools Barometric Formula Explanation If we let \(\eta\) be the number of molecules per unit volume, \(\eta. In contrast to incompressible fluids, the density of gases changes as a function of the applied pressure. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! The development of the barometric formula. Barometric Formula Explanation.
From slidetodoc.com
Lecture 4 Barometric formula and the Boltzmann equation Barometric Formula Explanation The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! Either of the latter relationships is frequently called the barometric formula. If the pressure is given in millimeters of mercury \(\left( \text{mmhg} \right),\) the barometric formula is written in the form: It is used to model.. Barometric Formula Explanation.
From www.studypool.com
SOLUTION Derivation of Barometric Equation Studypool Barometric Formula Explanation The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the. If we let \(\eta\) be the number of molecules per unit volume, \(\eta. It is used to model. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some. Barometric Formula Explanation.
From www.slideserve.com
PPT Lecture 5 Barometric formula and the Boltzmann equation Barometric Formula Explanation In contrast to incompressible fluids, the density of gases changes as a function of the applied pressure. Either of the latter relationships is frequently called the barometric formula. It is used to model. If we let \(\eta\) be the number of molecules per unit volume, \(\eta. The development of the barometric formula makes use of a number of concepts from. Barometric Formula Explanation.