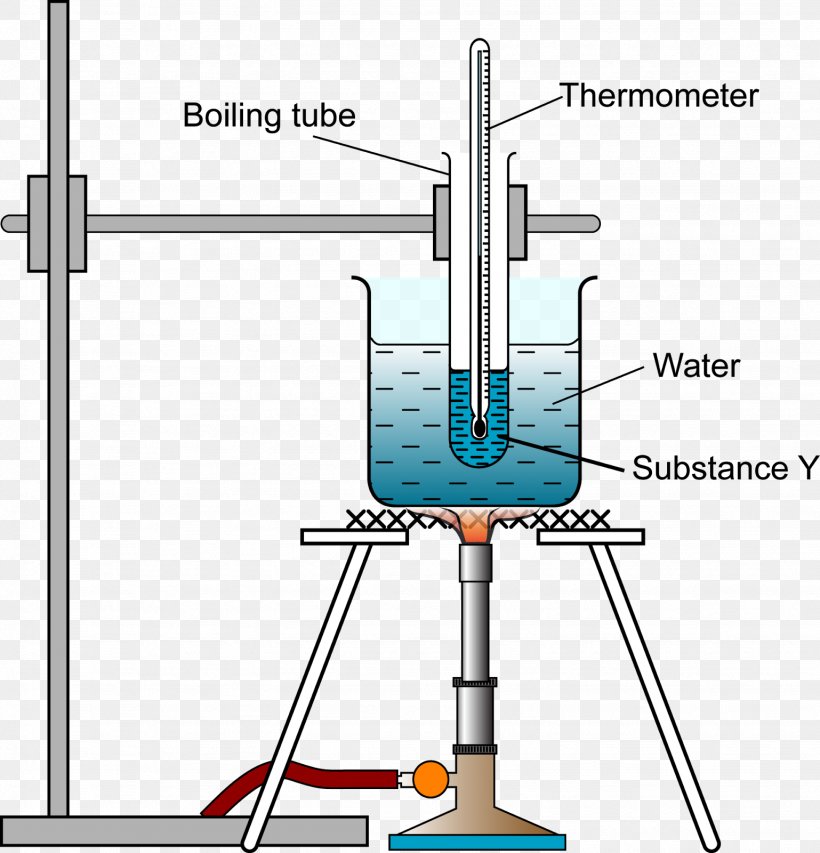
Boiling Point Of Liquid Solution . The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the temperature at which its vapor. The boiling point is that the temperature at which the pressure is exerted upon a liquid by the environment is equalled by the pressure exerted by the vapour of the liquid. Normally when we boil a liquid, we do so at atmospheric pressure. A related property of solutions is that their boiling points are higher than the boiling point of the pure solvent. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. The formal definition in science is that boiling point is the temperature where the vapor pressure of a. For example, the boiling point of water at sea level is 100 °c or 212 °f. Boiling point and freezing point effects. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure.
from animalia-life.club
Normally when we boil a liquid, we do so at atmospheric pressure. The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the temperature at which its vapor. For example, the boiling point of water at sea level is 100 °c or 212 °f. Boiling point and freezing point effects. A related property of solutions is that their boiling points are higher than the boiling point of the pure solvent. The boiling point is that the temperature at which the pressure is exerted upon a liquid by the environment is equalled by the pressure exerted by the vapour of the liquid. The formal definition in science is that boiling point is the temperature where the vapor pressure of a. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is.
Melting Point Apparatus Diagram
Boiling Point Of Liquid Solution Normally when we boil a liquid, we do so at atmospheric pressure. The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the temperature at which its vapor. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The formal definition in science is that boiling point is the temperature where the vapor pressure of a. The boiling point is that the temperature at which the pressure is exerted upon a liquid by the environment is equalled by the pressure exerted by the vapour of the liquid. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. A related property of solutions is that their boiling points are higher than the boiling point of the pure solvent. Boiling point and freezing point effects. For example, the boiling point of water at sea level is 100 °c or 212 °f. Normally when we boil a liquid, we do so at atmospheric pressure.
From www.chegg.com
Solved A certain liquid X has a normal boiling point of Boiling Point Of Liquid Solution For example, the boiling point of water at sea level is 100 °c or 212 °f. The formal definition in science is that boiling point is the temperature where the vapor pressure of a. A related property of solutions is that their boiling points are higher than the boiling point of the pure solvent. Normally when we boil a liquid,. Boiling Point Of Liquid Solution.
From www.pinterest.com
The Effect of Molecular Surface on the Boiling PointBoiling point Boiling Point Of Liquid Solution A related property of solutions is that their boiling points are higher than the boiling point of the pure solvent. The formal definition in science is that boiling point is the temperature where the vapor pressure of a. The boiling point is that the temperature at which the pressure is exerted upon a liquid by the environment is equalled by. Boiling Point Of Liquid Solution.
From www.youtube.com
Determine the Boiling Point of a Given Organic Liquid, Chemistry Boiling Point Of Liquid Solution Normally when we boil a liquid, we do so at atmospheric pressure. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. For example, the boiling point of water at sea level is 100 °c or 212 °f. If this pressure is the standard pressure of. Boiling Point Of Liquid Solution.
From blog.thermoworks.com
High Altitude and Its Effects on Cooking Boiling Point Of Liquid Solution The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the temperature at which its vapor. A related property of solutions is that their boiling points are higher than the boiling point of the pure solvent. The boiling point. Boiling Point Of Liquid Solution.
From www.studypool.com
SOLUTION Determination of boiling point of liquid organic compound Boiling Point Of Liquid Solution If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. Normally when we boil a liquid, we do so at atmospheric pressure. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. A related property. Boiling Point Of Liquid Solution.
From www.pinterest.com
Chemistry Lab The Boiling Points Of Liquids Chemistry labs, Science Boiling Point Of Liquid Solution The boiling point is that the temperature at which the pressure is exerted upon a liquid by the environment is equalled by the pressure exerted by the vapour of the liquid. The formal definition in science is that boiling point is the temperature where the vapor pressure of a. A related property of solutions is that their boiling points are. Boiling Point Of Liquid Solution.
From www.chegg.com
Solved The reported boiling point for a liquid is 200oC at 1 Boiling Point Of Liquid Solution The formal definition in science is that boiling point is the temperature where the vapor pressure of a. Normally when we boil a liquid, we do so at atmospheric pressure. The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid. Boiling Point Of Liquid Solution.
From cristor.dz
acceptabil schiță încheietura liquid nitrogen boiling point vs pressure Boiling Point Of Liquid Solution A related property of solutions is that their boiling points are higher than the boiling point of the pure solvent. The formal definition in science is that boiling point is the temperature where the vapor pressure of a. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. Boiling. Boiling Point Of Liquid Solution.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs Boiling Point Of Liquid Solution The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the temperature at which its vapor. The boiling point is that the temperature at which the pressure is exerted upon a liquid by the environment is equalled by the. Boiling Point Of Liquid Solution.
From www.northerngrafics.se
Perícia equilibrado Operação possível freezing and boiling point of Boiling Point Of Liquid Solution The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Normally when we boil a liquid, we do so at atmospheric pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. The formal definition. Boiling Point Of Liquid Solution.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock Boiling Point Of Liquid Solution The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. The formal definition in science is that boiling point is the temperature where the vapor. Boiling Point Of Liquid Solution.
From www.coursehero.com
[Solved] Four liquids are described in the table below. Use the second Boiling Point Of Liquid Solution The boiling point is that the temperature at which the pressure is exerted upon a liquid by the environment is equalled by the pressure exerted by the vapour of the liquid. A related property of solutions is that their boiling points are higher than the boiling point of the pure solvent. If this pressure is the standard pressure of 1. Boiling Point Of Liquid Solution.
From brainly.com
This graph shows the melting and boiling points of the alkali metals Boiling Point Of Liquid Solution If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. The formal definition in science is that boiling point is the temperature where the vapor pressure of a. Normally when we boil a liquid, we do so at atmospheric pressure. The boiling point is defined as the temperature at. Boiling Point Of Liquid Solution.
From studylibplimsoll.z21.web.core.windows.net
Blood Boils At What Temperature Boiling Point Of Liquid Solution If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. Boiling point and freezing point effects. Normally when we boil a liquid, we do so at atmospheric pressure. A related property of solutions is that their boiling points are higher than the boiling point of the pure solvent. The. Boiling Point Of Liquid Solution.
From abvcurve.com
Boiling Points of liquids and its effect on production of spirits Boiling Point Of Liquid Solution Normally when we boil a liquid, we do so at atmospheric pressure. A related property of solutions is that their boiling points are higher than the boiling point of the pure solvent. The boiling point is that the temperature at which the pressure is exerted upon a liquid by the environment is equalled by the pressure exerted by the vapour. Boiling Point Of Liquid Solution.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy Boiling Point Of Liquid Solution The formal definition in science is that boiling point is the temperature where the vapor pressure of a. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Normally when we boil a liquid, we do so at atmospheric pressure. A related property of solutions is. Boiling Point Of Liquid Solution.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Boiling Point Of Liquid Solution Normally when we boil a liquid, we do so at atmospheric pressure. For example, the boiling point of water at sea level is 100 °c or 212 °f. Boiling point and freezing point effects. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The boiling. Boiling Point Of Liquid Solution.
From www.pinterest.com
Chemistry Lab The Boiling Points Of Liquids Chemistry labs, High Boiling Point Of Liquid Solution The boiling point is that the temperature at which the pressure is exerted upon a liquid by the environment is equalled by the pressure exerted by the vapour of the liquid. For example, the boiling point of water at sea level is 100 °c or 212 °f. The formal definition in science is that boiling point is the temperature where. Boiling Point Of Liquid Solution.
From brainly.com
Arrange the boiling points of the aqueous solutions, relative to pure Boiling Point Of Liquid Solution If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the temperature at which its vapor. A related. Boiling Point Of Liquid Solution.
From brainly.com
Four liquids are described in the table below. Use the second column of Boiling Point Of Liquid Solution A related property of solutions is that their boiling points are higher than the boiling point of the pure solvent. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The boiling point of a liquid is the temperature at which its vapor pressure is equal. Boiling Point Of Liquid Solution.
From marshallkruwmathis.blogspot.com
What is the Boiling Point of Water MarshallkruwMathis Boiling Point Of Liquid Solution The boiling point is that the temperature at which the pressure is exerted upon a liquid by the environment is equalled by the pressure exerted by the vapour of the liquid. For example, the boiling point of water at sea level is 100 °c or 212 °f. Boiling point and freezing point effects. The boiling point of a liquid is. Boiling Point Of Liquid Solution.
From www.youtube.com
Boiling Point Of Liquids and The Factors Which Effect the Boiling Point Boiling Point Of Liquid Solution The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The formal definition in science is that boiling point is the temperature where the vapor pressure of a. A related property of solutions is that their boiling points are higher than the boiling point of the. Boiling Point Of Liquid Solution.
From www.teachoo.com
What is the Difference between Evaporation and Boiling? Class 9 Boiling Point Of Liquid Solution The formal definition in science is that boiling point is the temperature where the vapor pressure of a. The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the temperature at which its vapor. If this pressure is the. Boiling Point Of Liquid Solution.
From www.toppr.com
The normal boiling point of a liquid is that temperature at which Boiling Point Of Liquid Solution The formal definition in science is that boiling point is the temperature where the vapor pressure of a. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. The boiling point is that the temperature at which the pressure is exerted upon a liquid by the environment is equalled. Boiling Point Of Liquid Solution.
From www.greelane.com
Pochopte, čo znamená varenie v chémii Boiling Point Of Liquid Solution The formal definition in science is that boiling point is the temperature where the vapor pressure of a. The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the temperature at which its vapor. For example, the boiling point. Boiling Point Of Liquid Solution.
From edu.thainfo.info
Boiling Point คือ อะไร ความรู้และความเข้าใจ Boiling Point Of Liquid Solution The boiling point is that the temperature at which the pressure is exerted upon a liquid by the environment is equalled by the pressure exerted by the vapour of the liquid. The formal definition in science is that boiling point is the temperature where the vapor pressure of a. A related property of solutions is that their boiling points are. Boiling Point Of Liquid Solution.
From www.cutco.com
Technique Boiling and Simmering Boiling Point Of Liquid Solution If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. Normally when we boil a liquid, we do so at atmospheric pressure. For example, the boiling point of water at sea level is 100 °c or 212 °f. Boiling point and freezing point effects. The boiling point is defined. Boiling Point Of Liquid Solution.
From studylib.net
Boiling Point of Liquids Boiling Point Of Liquid Solution A related property of solutions is that their boiling points are higher than the boiling point of the pure solvent. Boiling point and freezing point effects. For example, the boiling point of water at sea level is 100 °c or 212 °f. Normally when we boil a liquid, we do so at atmospheric pressure. The formal definition in science is. Boiling Point Of Liquid Solution.
From www.thoughtco.com
Normal Boiling Point Definition (Chemistry) Boiling Point Of Liquid Solution The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The formal definition in science is that boiling point is the temperature where the vapor pressure of a. For example, the boiling point of water at sea level is 100 °c or 212 °f. The boiling. Boiling Point Of Liquid Solution.
From www.studypool.com
SOLUTION Determine the boiling point of an organic compound Studypool Boiling Point Of Liquid Solution The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. The boiling point is that the temperature at which the pressure is exerted upon a. Boiling Point Of Liquid Solution.
From animalia-life.club
Melting Point Apparatus Diagram Boiling Point Of Liquid Solution The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the temperature at which its vapor. For example, the boiling point of water at sea level is 100 °c or 212 °f. Boiling point and freezing point effects. The. Boiling Point Of Liquid Solution.
From www.chegg.com
Solved The boiling point of liquid A is 75.5°C at 1atm Boiling Point Of Liquid Solution A related property of solutions is that their boiling points are higher than the boiling point of the pure solvent. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. For example, the boiling point of water at sea level is 100 °c or 212 °f.. Boiling Point Of Liquid Solution.
From ar.inspiredpencil.com
Boiling Point Of Water Examples Boiling Point Of Liquid Solution The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. The boiling point of a liquid is the temperature at which its vapor pressure is. Boiling Point Of Liquid Solution.
From www.youtube.com
The rise in boiling point of a solution containing `1.8g` glucose in Boiling Point Of Liquid Solution If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. Normally when we boil a liquid, we do so at atmospheric pressure. Boiling point and freezing point effects. A related property of solutions is that their boiling points are higher than the boiling point of the pure solvent. The. Boiling Point Of Liquid Solution.
From facts.net
20 Fascinating Facts About Boiling Point Boiling Point Of Liquid Solution If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the temperature at which its vapor. The formal. Boiling Point Of Liquid Solution.