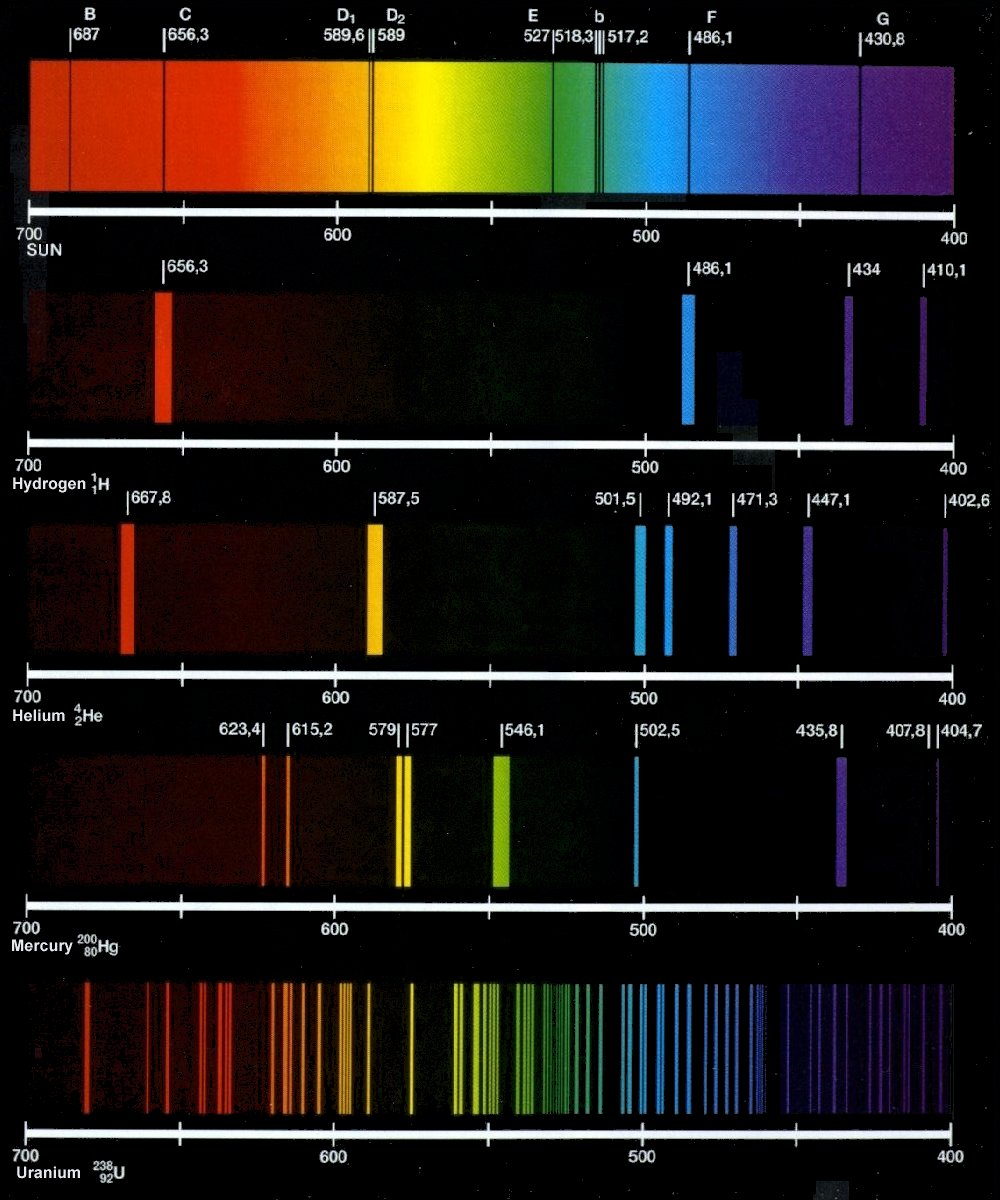
What Kind Of Spectrum Does Incandescent Light Produce . A continuous spectrum (the spectrum of white light) an incandescent light bulb uses electric resistance to produce heat which makes light. An incandescent light bulb has a continuous spectrum because it produces light by heating a metal filament to a high temperature. Thomas edison tried thousands of. When the metal filament is heated, it. This process creates a continuous spectrum of light that closely resembles natural. When hydrogen gas is placed. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Incandescent bulbs emit light by heating a filament using electricity, this would lead to a continuous spectrum according to. This means that they produce light across the entire visible spectrum, creating a. Incandescent bulbs emit a unique spectrum of light known as a thermal or continuous spectrum.
from educationgrafts.z21.web.core.windows.net
The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. An incandescent light bulb has a continuous spectrum because it produces light by heating a metal filament to a high temperature. Incandescent bulbs emit light by heating a filament using electricity, this would lead to a continuous spectrum according to. A continuous spectrum (the spectrum of white light) an incandescent light bulb uses electric resistance to produce heat which makes light. Incandescent bulbs emit a unique spectrum of light known as a thermal or continuous spectrum. Thomas edison tried thousands of. This process creates a continuous spectrum of light that closely resembles natural. When hydrogen gas is placed. When the metal filament is heated, it. This means that they produce light across the entire visible spectrum, creating a.
What Is An Emission Line Spectrum
What Kind Of Spectrum Does Incandescent Light Produce When hydrogen gas is placed. This process creates a continuous spectrum of light that closely resembles natural. Incandescent bulbs emit a unique spectrum of light known as a thermal or continuous spectrum. When the metal filament is heated, it. A continuous spectrum (the spectrum of white light) an incandescent light bulb uses electric resistance to produce heat which makes light. Incandescent bulbs emit light by heating a filament using electricity, this would lead to a continuous spectrum according to. Thomas edison tried thousands of. An incandescent light bulb has a continuous spectrum because it produces light by heating a metal filament to a high temperature. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed. This means that they produce light across the entire visible spectrum, creating a.
From quickandeasylighting.com
An Incandescent Light Bulb Produces What Kind of Visible Spectrum? What Kind Of Spectrum Does Incandescent Light Produce Incandescent bulbs emit a unique spectrum of light known as a thermal or continuous spectrum. An incandescent light bulb has a continuous spectrum because it produces light by heating a metal filament to a high temperature. This process creates a continuous spectrum of light that closely resembles natural. The emission spectrum (or line spectrum) of a chemical element is the. What Kind Of Spectrum Does Incandescent Light Produce.
From brightestlumen.com
What Kind of Spectrum Does an Incandescent Light Bulb Emit? Brightest What Kind Of Spectrum Does Incandescent Light Produce Thomas edison tried thousands of. Incandescent bulbs emit light by heating a filament using electricity, this would lead to a continuous spectrum according to. When hydrogen gas is placed. This means that they produce light across the entire visible spectrum, creating a. A continuous spectrum (the spectrum of white light) an incandescent light bulb uses electric resistance to produce heat. What Kind Of Spectrum Does Incandescent Light Produce.
From www.researchgate.net
Comparison of spectrum readings of the 3 different lighting sources What Kind Of Spectrum Does Incandescent Light Produce An incandescent light bulb has a continuous spectrum because it produces light by heating a metal filament to a high temperature. Thomas edison tried thousands of. Incandescent bulbs emit a unique spectrum of light known as a thermal or continuous spectrum. This process creates a continuous spectrum of light that closely resembles natural. When hydrogen gas is placed. Incandescent bulbs. What Kind Of Spectrum Does Incandescent Light Produce.
From lednique.com
Spectral_power_distribution_of_a_25_W_incandescent_light_bulb LEDnique What Kind Of Spectrum Does Incandescent Light Produce When hydrogen gas is placed. This process creates a continuous spectrum of light that closely resembles natural. Thomas edison tried thousands of. A continuous spectrum (the spectrum of white light) an incandescent light bulb uses electric resistance to produce heat which makes light. Incandescent bulbs emit light by heating a filament using electricity, this would lead to a continuous spectrum. What Kind Of Spectrum Does Incandescent Light Produce.
From buildgreennh.com
19 Types of Light Bulbs and Why You Should Care What Kind Of Spectrum Does Incandescent Light Produce When hydrogen gas is placed. A continuous spectrum (the spectrum of white light) an incandescent light bulb uses electric resistance to produce heat which makes light. When the metal filament is heated, it. Thomas edison tried thousands of. An incandescent light bulb has a continuous spectrum because it produces light by heating a metal filament to a high temperature. Incandescent. What Kind Of Spectrum Does Incandescent Light Produce.
From www.researchgate.net
1 Diagram of the light's spectrum, showing the What Kind Of Spectrum Does Incandescent Light Produce This means that they produce light across the entire visible spectrum, creating a. Incandescent bulbs emit a unique spectrum of light known as a thermal or continuous spectrum. Thomas edison tried thousands of. When the metal filament is heated, it. This process creates a continuous spectrum of light that closely resembles natural. A continuous spectrum (the spectrum of white light). What Kind Of Spectrum Does Incandescent Light Produce.
From www.researchgate.net
Emission spectra of different light sources (a) incandescent tungsten What Kind Of Spectrum Does Incandescent Light Produce The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When the metal filament is heated, it. Incandescent bulbs emit light by heating a filament using electricity, this would lead to a continuous spectrum according to. Thomas edison tried thousands of. This process creates. What Kind Of Spectrum Does Incandescent Light Produce.
From americanwarmoms.org
Incandescent Light Bulb Spectrum What Kind Of Spectrum Does Incandescent Light Produce Incandescent bulbs emit light by heating a filament using electricity, this would lead to a continuous spectrum according to. This process creates a continuous spectrum of light that closely resembles natural. Incandescent bulbs emit a unique spectrum of light known as a thermal or continuous spectrum. An incandescent light bulb has a continuous spectrum because it produces light by heating. What Kind Of Spectrum Does Incandescent Light Produce.
From ar.inspiredpencil.com
Incandescent Light Spectrum Lines What Kind Of Spectrum Does Incandescent Light Produce Incandescent bulbs emit light by heating a filament using electricity, this would lead to a continuous spectrum according to. Incandescent bulbs emit a unique spectrum of light known as a thermal or continuous spectrum. This process creates a continuous spectrum of light that closely resembles natural. The emission spectrum (or line spectrum) of a chemical element is the unique pattern. What Kind Of Spectrum Does Incandescent Light Produce.
From brightestlumen.com
What Kind of Spectrum Does an Incandescent Light Bulb Emit? Brightest What Kind Of Spectrum Does Incandescent Light Produce This process creates a continuous spectrum of light that closely resembles natural. A continuous spectrum (the spectrum of white light) an incandescent light bulb uses electric resistance to produce heat which makes light. When the metal filament is heated, it. Incandescent bulbs emit light by heating a filament using electricity, this would lead to a continuous spectrum according to. An. What Kind Of Spectrum Does Incandescent Light Produce.
From educationgrafts.z21.web.core.windows.net
What Is An Emission Line Spectrum What Kind Of Spectrum Does Incandescent Light Produce An incandescent light bulb has a continuous spectrum because it produces light by heating a metal filament to a high temperature. When hydrogen gas is placed. A continuous spectrum (the spectrum of white light) an incandescent light bulb uses electric resistance to produce heat which makes light. When the metal filament is heated, it. The emission spectrum (or line spectrum). What Kind Of Spectrum Does Incandescent Light Produce.
From www.smorescience.com
Visible Light Spectrum Wavelengths Poster Free Download Smore Science What Kind Of Spectrum Does Incandescent Light Produce This means that they produce light across the entire visible spectrum, creating a. Incandescent bulbs emit light by heating a filament using electricity, this would lead to a continuous spectrum according to. This process creates a continuous spectrum of light that closely resembles natural. When hydrogen gas is placed. When the metal filament is heated, it. A continuous spectrum (the. What Kind Of Spectrum Does Incandescent Light Produce.
From gooderdle.com
What Kind Of Visible Spectrum Does An Incandescent Light Bulb Produce What Kind Of Spectrum Does Incandescent Light Produce Incandescent bulbs emit a unique spectrum of light known as a thermal or continuous spectrum. This process creates a continuous spectrum of light that closely resembles natural. Thomas edison tried thousands of. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. This means. What Kind Of Spectrum Does Incandescent Light Produce.
From slideplayer.com
Objectives To learn the Properties of light ppt download What Kind Of Spectrum Does Incandescent Light Produce When hydrogen gas is placed. This process creates a continuous spectrum of light that closely resembles natural. Incandescent bulbs emit light by heating a filament using electricity, this would lead to a continuous spectrum according to. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. What Kind Of Spectrum Does Incandescent Light Produce.
From imagine.gsfc.nasa.gov
Spectra Introduction What Kind Of Spectrum Does Incandescent Light Produce This process creates a continuous spectrum of light that closely resembles natural. Incandescent bulbs emit a unique spectrum of light known as a thermal or continuous spectrum. When hydrogen gas is placed. Incandescent bulbs emit light by heating a filament using electricity, this would lead to a continuous spectrum according to. When the metal filament is heated, it. The emission. What Kind Of Spectrum Does Incandescent Light Produce.
From americanwarmoms.org
Incandescent Light Bulb Visible Spectrum What Kind Of Spectrum Does Incandescent Light Produce This means that they produce light across the entire visible spectrum, creating a. A continuous spectrum (the spectrum of white light) an incandescent light bulb uses electric resistance to produce heat which makes light. An incandescent light bulb has a continuous spectrum because it produces light by heating a metal filament to a high temperature. The emission spectrum (or line. What Kind Of Spectrum Does Incandescent Light Produce.
From www.makegreatlight.com
Full spectrum light without expensive full spectrum bulbs What Kind Of Spectrum Does Incandescent Light Produce When the metal filament is heated, it. This process creates a continuous spectrum of light that closely resembles natural. When hydrogen gas is placed. Incandescent bulbs emit light by heating a filament using electricity, this would lead to a continuous spectrum according to. This means that they produce light across the entire visible spectrum, creating a. The emission spectrum (or. What Kind Of Spectrum Does Incandescent Light Produce.
From americanwarmoms.org
Incandescent Light Bulb Visible Spectrum What Kind Of Spectrum Does Incandescent Light Produce An incandescent light bulb has a continuous spectrum because it produces light by heating a metal filament to a high temperature. Thomas edison tried thousands of. A continuous spectrum (the spectrum of white light) an incandescent light bulb uses electric resistance to produce heat which makes light. Incandescent bulbs emit a unique spectrum of light known as a thermal or. What Kind Of Spectrum Does Incandescent Light Produce.
From americanwarmoms.org
An Incandescent Light Bulb Produces What Kind Of Visible Spectrum What Kind Of Spectrum Does Incandescent Light Produce This means that they produce light across the entire visible spectrum, creating a. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When the metal filament is heated, it. This process creates a continuous spectrum of light that closely resembles natural. A continuous. What Kind Of Spectrum Does Incandescent Light Produce.
From sciencenotes.org
Visible Light Spectrum Wavelengths and Colors What Kind Of Spectrum Does Incandescent Light Produce The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed. Thomas edison tried thousands of. An incandescent light bulb has a continuous spectrum because it produces light by heating a metal filament to a high temperature. This means that. What Kind Of Spectrum Does Incandescent Light Produce.
From lumenauthority.com
How Does a Incandescent Light Bulb Work? [StepbyStep] What Kind Of Spectrum Does Incandescent Light Produce Incandescent bulbs emit light by heating a filament using electricity, this would lead to a continuous spectrum according to. When hydrogen gas is placed. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. This means that they produce light across the entire visible. What Kind Of Spectrum Does Incandescent Light Produce.
From www.youtube.com
Spectra from Incandescent Filament, Discharge Tubes and Sunlight // HSC What Kind Of Spectrum Does Incandescent Light Produce Incandescent bulbs emit light by heating a filament using electricity, this would lead to a continuous spectrum according to. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. This means that they produce light across the entire visible spectrum, creating a. Thomas edison. What Kind Of Spectrum Does Incandescent Light Produce.
From americanwarmoms.org
What Kind Of Spectrum Does Incandescent Light Produce What Kind Of Spectrum Does Incandescent Light Produce Thomas edison tried thousands of. This process creates a continuous spectrum of light that closely resembles natural. A continuous spectrum (the spectrum of white light) an incandescent light bulb uses electric resistance to produce heat which makes light. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected. What Kind Of Spectrum Does Incandescent Light Produce.
From waves.neocities.org
The Spectrum What Kind Of Spectrum Does Incandescent Light Produce Incandescent bulbs emit light by heating a filament using electricity, this would lead to a continuous spectrum according to. A continuous spectrum (the spectrum of white light) an incandescent light bulb uses electric resistance to produce heat which makes light. This means that they produce light across the entire visible spectrum, creating a. The emission spectrum (or line spectrum) of. What Kind Of Spectrum Does Incandescent Light Produce.
From americanwarmoms.org
Incandescent Light Bulb Visible Spectrum What Kind Of Spectrum Does Incandescent Light Produce A continuous spectrum (the spectrum of white light) an incandescent light bulb uses electric resistance to produce heat which makes light. Incandescent bulbs emit light by heating a filament using electricity, this would lead to a continuous spectrum according to. When the metal filament is heated, it. This means that they produce light across the entire visible spectrum, creating a.. What Kind Of Spectrum Does Incandescent Light Produce.
From americanwarmoms.org
Why Does Incandescent Light Produce A Continuous Spectrum What Kind Of Spectrum Does Incandescent Light Produce This process creates a continuous spectrum of light that closely resembles natural. An incandescent light bulb has a continuous spectrum because it produces light by heating a metal filament to a high temperature. A continuous spectrum (the spectrum of white light) an incandescent light bulb uses electric resistance to produce heat which makes light. This means that they produce light. What Kind Of Spectrum Does Incandescent Light Produce.
From ar.inspiredpencil.com
Incandescent Light Spectrum What Kind Of Spectrum Does Incandescent Light Produce When hydrogen gas is placed. Thomas edison tried thousands of. When the metal filament is heated, it. This means that they produce light across the entire visible spectrum, creating a. Incandescent bulbs emit light by heating a filament using electricity, this would lead to a continuous spectrum according to. The emission spectrum (or line spectrum) of a chemical element is. What Kind Of Spectrum Does Incandescent Light Produce.
From www.astronomy.ohio-state.edu
Lecture 24 Matter & Light What Kind Of Spectrum Does Incandescent Light Produce Thomas edison tried thousands of. This means that they produce light across the entire visible spectrum, creating a. An incandescent light bulb has a continuous spectrum because it produces light by heating a metal filament to a high temperature. This process creates a continuous spectrum of light that closely resembles natural. Incandescent bulbs emit light by heating a filament using. What Kind Of Spectrum Does Incandescent Light Produce.
From www.urbanvine.co
What Are The Main Types of Grow Lights? (A Simple Guide) What Kind Of Spectrum Does Incandescent Light Produce An incandescent light bulb has a continuous spectrum because it produces light by heating a metal filament to a high temperature. Incandescent bulbs emit light by heating a filament using electricity, this would lead to a continuous spectrum according to. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element. What Kind Of Spectrum Does Incandescent Light Produce.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts What Kind Of Spectrum Does Incandescent Light Produce A continuous spectrum (the spectrum of white light) an incandescent light bulb uses electric resistance to produce heat which makes light. Thomas edison tried thousands of. When hydrogen gas is placed. An incandescent light bulb has a continuous spectrum because it produces light by heating a metal filament to a high temperature. Incandescent bulbs emit light by heating a filament. What Kind Of Spectrum Does Incandescent Light Produce.
From davisexcleduess.blogspot.com
Incandescent Light Bulb Emission Spectrum Davis Excleduess What Kind Of Spectrum Does Incandescent Light Produce This means that they produce light across the entire visible spectrum, creating a. Incandescent bulbs emit light by heating a filament using electricity, this would lead to a continuous spectrum according to. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Incandescent bulbs. What Kind Of Spectrum Does Incandescent Light Produce.
From americanwarmoms.org
Incandescent Light Bulb Visible Spectrum What Kind Of Spectrum Does Incandescent Light Produce An incandescent light bulb has a continuous spectrum because it produces light by heating a metal filament to a high temperature. When the metal filament is heated, it. Incandescent bulbs emit light by heating a filament using electricity, this would lead to a continuous spectrum according to. The emission spectrum (or line spectrum) of a chemical element is the unique. What Kind Of Spectrum Does Incandescent Light Produce.
From www.livescience.com
Lightbulbs Incandescent, Fluorescent, LED (Infographic) Live Science What Kind Of Spectrum Does Incandescent Light Produce Incandescent bulbs emit a unique spectrum of light known as a thermal or continuous spectrum. Thomas edison tried thousands of. An incandescent light bulb has a continuous spectrum because it produces light by heating a metal filament to a high temperature. Incandescent bulbs emit light by heating a filament using electricity, this would lead to a continuous spectrum according to.. What Kind Of Spectrum Does Incandescent Light Produce.
From animalia-life.club
Incandescent Light Spectrum Vs Fluorescent What Kind Of Spectrum Does Incandescent Light Produce An incandescent light bulb has a continuous spectrum because it produces light by heating a metal filament to a high temperature. When the metal filament is heated, it. When hydrogen gas is placed. A continuous spectrum (the spectrum of white light) an incandescent light bulb uses electric resistance to produce heat which makes light. Incandescent bulbs emit light by heating. What Kind Of Spectrum Does Incandescent Light Produce.
From americanwarmoms.org
Incandescent Light Bulb Visible Spectrum What Kind Of Spectrum Does Incandescent Light Produce This means that they produce light across the entire visible spectrum, creating a. When hydrogen gas is placed. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Incandescent bulbs emit a unique spectrum of light known as a thermal or continuous spectrum. When. What Kind Of Spectrum Does Incandescent Light Produce.