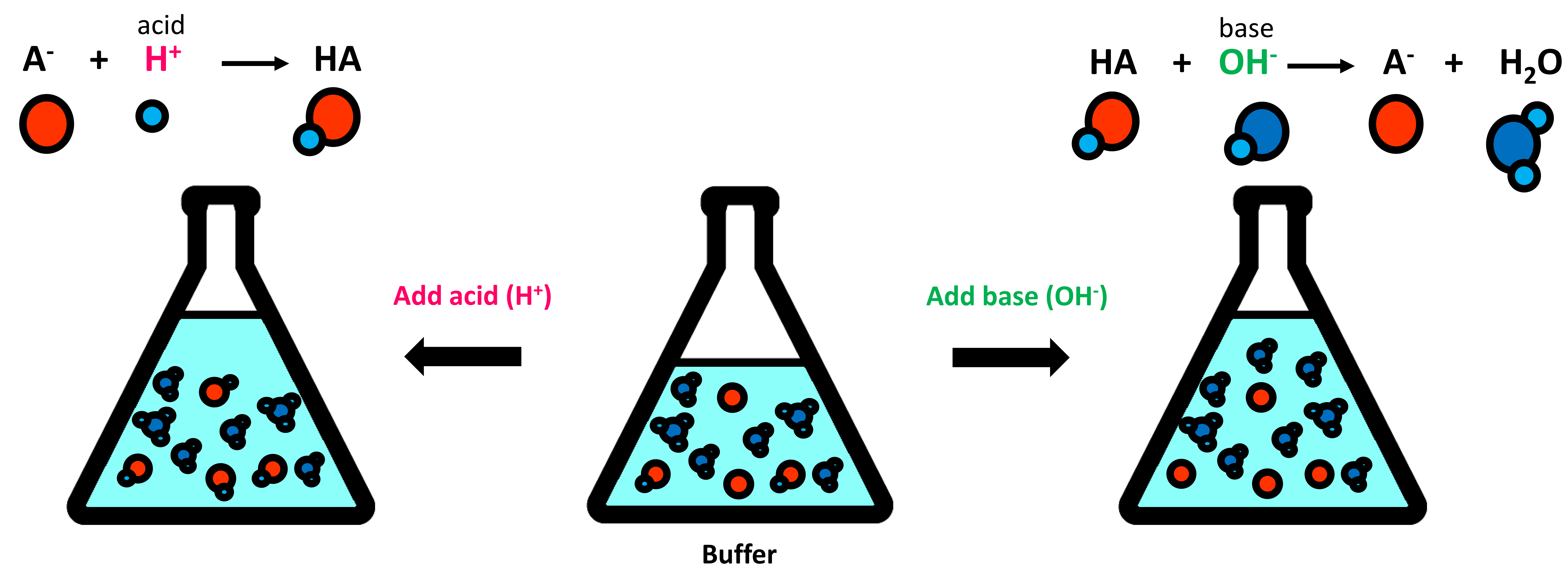
Biological Examples Of Buffer . What are examples of biological buffers? A buffer is a solution that maintains the. There are various biological buffers designed to maintain physiological ph, especially for laboratory use. Buffers do so by being composed of certain pairs of solutes: A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Buffer definition and examples in chemistry. Mixing precalculated amounts of monobasic and dibasic sodium phosphates. Biological buffers is shared under a not declared license and was authored, remixed, and/or curated by libretexts. A buffer solution resists a change in ph from the addition of a small amount of acid or base. In this way, a biological buffer. A few examples of acidic buffers are shown above. Rom 6.55 to 8.55.use stock solutions to prepare phosphate buffers. Either a weak acid plus its conjugate base or a weak base plus its conjugate acid. The experimental group would have a lower ph and thus, a higher [h+].
from deporecipe.co
Either a weak acid plus its conjugate base or a weak base plus its conjugate acid. A buffer is a solution that maintains the. Biological buffers is shared under a not declared license and was authored, remixed, and/or curated by libretexts. Mixing precalculated amounts of monobasic and dibasic sodium phosphates. In this way, a biological buffer. Buffer definition and examples in chemistry. The experimental group would have a lower ph and thus, a higher [h+]. What are examples of biological buffers? Buffers do so by being composed of certain pairs of solutes: There are various biological buffers designed to maintain physiological ph, especially for laboratory use.
10x Protein Running Buffer Recipe Deporecipe.co
Biological Examples Of Buffer What are examples of biological buffers? Either a weak acid plus its conjugate base or a weak base plus its conjugate acid. In this way, a biological buffer. Buffer definition and examples in chemistry. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Biological buffers is shared under a not declared license and was authored, remixed, and/or curated by libretexts. Mixing precalculated amounts of monobasic and dibasic sodium phosphates. What are examples of biological buffers? A buffer is a solution that maintains the. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffers do so by being composed of certain pairs of solutes: Rom 6.55 to 8.55.use stock solutions to prepare phosphate buffers. The experimental group would have a lower ph and thus, a higher [h+]. There are various biological buffers designed to maintain physiological ph, especially for laboratory use. A few examples of acidic buffers are shown above.
From dandkmotorsports.com
Hepes Buffer Recipe Ph 7 2 Dandk Organizer Biological Examples Of Buffer A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Mixing precalculated amounts of monobasic and dibasic sodium phosphates. Buffer definition and examples in chemistry. There are various biological buffers designed to maintain physiological ph, especially for laboratory use. In this way, a biological buffer. The experimental group would have a lower ph and thus,. Biological Examples Of Buffer.
From www.expii.com
Buffers — Definition & Overview Expii Biological Examples Of Buffer In this way, a biological buffer. Buffer definition and examples in chemistry. Biological buffers is shared under a not declared license and was authored, remixed, and/or curated by libretexts. A buffer solution resists a change in ph from the addition of a small amount of acid or base. The experimental group would have a lower ph and thus, a higher. Biological Examples Of Buffer.
From animalia-life.club
Phosphate Buffer System Equation Biological Examples Of Buffer In this way, a biological buffer. A few examples of acidic buffers are shown above. There are various biological buffers designed to maintain physiological ph, especially for laboratory use. Rom 6.55 to 8.55.use stock solutions to prepare phosphate buffers. Either a weak acid plus its conjugate base or a weak base plus its conjugate acid. The experimental group would have. Biological Examples Of Buffer.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation Biological Examples Of Buffer The experimental group would have a lower ph and thus, a higher [h+]. Either a weak acid plus its conjugate base or a weak base plus its conjugate acid. Biological buffers is shared under a not declared license and was authored, remixed, and/or curated by libretexts. Buffers do so by being composed of certain pairs of solutes: A buffer solution. Biological Examples Of Buffer.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its Biological Examples Of Buffer Either a weak acid plus its conjugate base or a weak base plus its conjugate acid. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. A few examples of acidic buffers are shown above. A buffer is a solution that maintains the. A buffer solution resists a change in ph from the addition of. Biological Examples Of Buffer.
From www.youtube.com
Buffer action in the blood YouTube Biological Examples Of Buffer A buffer is a solution that maintains the. There are various biological buffers designed to maintain physiological ph, especially for laboratory use. A few examples of acidic buffers are shown above. Buffers do so by being composed of certain pairs of solutes: A buffer solution resists a change in ph from the addition of a small amount of acid or. Biological Examples Of Buffer.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Biological Examples Of Buffer In this way, a biological buffer. A few examples of acidic buffers are shown above. Either a weak acid plus its conjugate base or a weak base plus its conjugate acid. Biological buffers is shared under a not declared license and was authored, remixed, and/or curated by libretexts. A biological buffer is an organic substance that has a neutralizing effect. Biological Examples Of Buffer.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Biological Examples Of Buffer A buffer is a solution that maintains the. Buffers do so by being composed of certain pairs of solutes: Mixing precalculated amounts of monobasic and dibasic sodium phosphates. In this way, a biological buffer. A buffer solution resists a change in ph from the addition of a small amount of acid or base. There are various biological buffers designed to. Biological Examples Of Buffer.
From www.youtube.com
Chemical Buffers protein buffer, phosphate buffer system and Biological Examples Of Buffer A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. A few examples of acidic buffers are shown above. Either a weak acid plus its conjugate base or a weak base plus its conjugate acid. Buffer definition and examples in chemistry. A buffer is a solution that maintains the. Biological buffers is shared under a. Biological Examples Of Buffer.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts Biological Examples Of Buffer A buffer is a solution that maintains the. What are examples of biological buffers? Buffer definition and examples in chemistry. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Mixing precalculated amounts of monobasic and dibasic sodium phosphates. The experimental group would have a lower ph and thus, a higher [h+]. In this way,. Biological Examples Of Buffer.
From www.researchgate.net
Examples of buffer compounds divided into accepted physiological Biological Examples Of Buffer Buffers do so by being composed of certain pairs of solutes: In this way, a biological buffer. A few examples of acidic buffers are shown above. Buffer definition and examples in chemistry. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. A buffer is a solution that maintains the. The experimental group would have. Biological Examples Of Buffer.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Biological Examples Of Buffer There are various biological buffers designed to maintain physiological ph, especially for laboratory use. A few examples of acidic buffers are shown above. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Rom 6.55 to 8.55.use stock solutions to prepare phosphate buffers. The experimental group would have a lower ph. Biological Examples Of Buffer.
From www.slideshare.net
Buffer system Biological Examples Of Buffer Buffer definition and examples in chemistry. In this way, a biological buffer. There are various biological buffers designed to maintain physiological ph, especially for laboratory use. A buffer is a solution that maintains the. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. What are examples of biological buffers? A buffer solution resists a. Biological Examples Of Buffer.
From www.studocu.com
Biological Buffer Systems BIOLOGICAL BUFFER SYSTEMS Almost every Biological Examples Of Buffer In this way, a biological buffer. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Mixing precalculated amounts of monobasic and dibasic sodium phosphates. Buffer definition and examples in chemistry. The experimental group would have a lower ph and thus, a higher [h+]. What are examples of biological buffers? A few examples of acidic. Biological Examples Of Buffer.
From www.slideserve.com
PPT Acids and Bases and Buffers PowerPoint Presentation, free Biological Examples Of Buffer In this way, a biological buffer. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. A few examples of acidic buffers are shown above. Buffers do so by being composed of certain pairs of solutes: The experimental group would have a lower ph and thus, a higher [h+]. A buffer solution resists a change. Biological Examples Of Buffer.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high Biological Examples Of Buffer Biological buffers is shared under a not declared license and was authored, remixed, and/or curated by libretexts. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Rom 6.55 to 8.55.use stock solutions to prepare phosphate buffers. A few examples of acidic buffers are shown above. Mixing precalculated amounts of monobasic and dibasic sodium phosphates.. Biological Examples Of Buffer.
From kyvokedymolyb.modellervefiyatlar.com
Acid Base Buffers Biological Examples Of Buffer A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Rom 6.55 to 8.55.use stock solutions to prepare phosphate buffers. Buffer definition and examples in chemistry. A buffer is a solution that maintains the. Mixing precalculated amounts of monobasic and dibasic sodium phosphates. What are examples of biological buffers? A few examples of acidic buffers. Biological Examples Of Buffer.
From www.slideshare.net
Buffers in chemical analysis, types of buffers Biological Examples Of Buffer A few examples of acidic buffers are shown above. The experimental group would have a lower ph and thus, a higher [h+]. A buffer is a solution that maintains the. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Either a weak acid plus its conjugate base or a weak. Biological Examples Of Buffer.
From study.com
Buffer System in Chemistry Definition & Overview Video & Lesson Biological Examples Of Buffer Either a weak acid plus its conjugate base or a weak base plus its conjugate acid. There are various biological buffers designed to maintain physiological ph, especially for laboratory use. Rom 6.55 to 8.55.use stock solutions to prepare phosphate buffers. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Mixing precalculated amounts of monobasic. Biological Examples Of Buffer.
From www.slideserve.com
PPT Water & Buffers PowerPoint Presentation, free download ID3886735 Biological Examples Of Buffer Either a weak acid plus its conjugate base or a weak base plus its conjugate acid. A few examples of acidic buffers are shown above. In this way, a biological buffer. Biological buffers is shared under a not declared license and was authored, remixed, and/or curated by libretexts. A buffer is a solution that maintains the. Rom 6.55 to 8.55.use. Biological Examples Of Buffer.
From magmastory.blogspot.com
Which Of The Following Represents A Buffer System? magmastory Biological Examples Of Buffer Buffers do so by being composed of certain pairs of solutes: There are various biological buffers designed to maintain physiological ph, especially for laboratory use. In this way, a biological buffer. The experimental group would have a lower ph and thus, a higher [h+]. Biological buffers is shared under a not declared license and was authored, remixed, and/or curated by. Biological Examples Of Buffer.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 Biological Examples Of Buffer Buffers do so by being composed of certain pairs of solutes: The experimental group would have a lower ph and thus, a higher [h+]. Buffer definition and examples in chemistry. Rom 6.55 to 8.55.use stock solutions to prepare phosphate buffers. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Either. Biological Examples Of Buffer.
From www.brainkart.com
Buffers Biological Examples Of Buffer A buffer is a solution that maintains the. In this way, a biological buffer. A few examples of acidic buffers are shown above. Either a weak acid plus its conjugate base or a weak base plus its conjugate acid. Biological buffers is shared under a not declared license and was authored, remixed, and/or curated by libretexts. What are examples of. Biological Examples Of Buffer.
From www.slideshare.net
Buffers in chemical analysis, types of buffers Biological Examples Of Buffer The experimental group would have a lower ph and thus, a higher [h+]. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. A few examples of acidic buffers are shown above. What are examples of biological buffers? Biological buffers is shared under a not declared license and was authored, remixed, and/or curated by libretexts.. Biological Examples Of Buffer.
From studylib.net
Buffer Solutions Biological Examples Of Buffer The experimental group would have a lower ph and thus, a higher [h+]. Either a weak acid plus its conjugate base or a weak base plus its conjugate acid. There are various biological buffers designed to maintain physiological ph, especially for laboratory use. In this way, a biological buffer. Mixing precalculated amounts of monobasic and dibasic sodium phosphates. A buffer. Biological Examples Of Buffer.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Biological Examples Of Buffer A buffer is a solution that maintains the. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Mixing precalculated amounts of monobasic and dibasic sodium phosphates. Either a weak acid plus its conjugate base or a weak base plus its conjugate acid. What are examples of biological buffers? In this way, a biological buffer.. Biological Examples Of Buffer.
From www.youtube.com
Buffers and pH Biology YouTube Biological Examples Of Buffer Rom 6.55 to 8.55.use stock solutions to prepare phosphate buffers. Buffer definition and examples in chemistry. Buffers do so by being composed of certain pairs of solutes: A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. A few examples of acidic buffers are shown above. Either a weak acid plus its conjugate base or. Biological Examples Of Buffer.
From www.pinterest.co.uk
Buffer Easy Science Chemistry education, Study chemistry, Chemistry Biological Examples Of Buffer Rom 6.55 to 8.55.use stock solutions to prepare phosphate buffers. A few examples of acidic buffers are shown above. The experimental group would have a lower ph and thus, a higher [h+]. What are examples of biological buffers? Mixing precalculated amounts of monobasic and dibasic sodium phosphates. A buffer solution resists a change in ph from the addition of a. Biological Examples Of Buffer.
From bitesizebio.com
How Do Buffers Work? An Easy Explaination For Biologists Biological Examples Of Buffer Rom 6.55 to 8.55.use stock solutions to prepare phosphate buffers. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. A few examples of acidic buffers are shown above. Buffers do so by being composed of certain pairs of solutes: Mixing precalculated amounts of monobasic and dibasic sodium phosphates. There are various biological buffers designed. Biological Examples Of Buffer.
From www.slideshare.net
Buffer system Biological Examples Of Buffer Rom 6.55 to 8.55.use stock solutions to prepare phosphate buffers. Buffers do so by being composed of certain pairs of solutes: A buffer is a solution that maintains the. The experimental group would have a lower ph and thus, a higher [h+]. There are various biological buffers designed to maintain physiological ph, especially for laboratory use. A biological buffer is. Biological Examples Of Buffer.
From www.youtube.com
BUFFER SOLUTIONS BUFFER CAPACITY APPLICATIONS OF BUFFERS IN Biological Examples Of Buffer A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Rom 6.55 to 8.55.use stock solutions to prepare phosphate buffers. What are examples of biological buffers? In this way, a biological buffer. Mixing precalculated amounts of monobasic and dibasic sodium phosphates. Buffer definition and examples in chemistry. A few examples of acidic buffers are shown. Biological Examples Of Buffer.
From deporecipe.co
10x Protein Running Buffer Recipe Deporecipe.co Biological Examples Of Buffer What are examples of biological buffers? Rom 6.55 to 8.55.use stock solutions to prepare phosphate buffers. A buffer is a solution that maintains the. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffer definition. Biological Examples Of Buffer.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Biological Examples Of Buffer Either a weak acid plus its conjugate base or a weak base plus its conjugate acid. Biological buffers is shared under a not declared license and was authored, remixed, and/or curated by libretexts. A few examples of acidic buffers are shown above. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. The experimental group. Biological Examples Of Buffer.
From sciencenotes.org
Buffer Definition and Examples in Chemistry Biological Examples Of Buffer In this way, a biological buffer. Buffers do so by being composed of certain pairs of solutes: A few examples of acidic buffers are shown above. Buffer definition and examples in chemistry. The experimental group would have a lower ph and thus, a higher [h+]. A buffer solution resists a change in ph from the addition of a small amount. Biological Examples Of Buffer.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Biological Examples Of Buffer Either a weak acid plus its conjugate base or a weak base plus its conjugate acid. There are various biological buffers designed to maintain physiological ph, especially for laboratory use. Buffer definition and examples in chemistry. Biological buffers is shared under a not declared license and was authored, remixed, and/or curated by libretexts. Mixing precalculated amounts of monobasic and dibasic. Biological Examples Of Buffer.