Can Water Store More Heat Than Air . A fast moving molecule (the cue ball) strikes a lattice of slow moving ice or water molecules. The cue ball rapidly decelerates (is cooled) as the. Water’s high heat capacity is a property caused by hydrogen bonding among water molecules. Because of this, it takes more energy to heat water than it does other substances. That means that water has a higher heat capacity—it can store more heat before changing in temperature. When heat is absorbed, hydrogen bonds are broken. It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release thermal energy. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Air has a heat capacity of about 1000 joules per kg per °k and a density of just 1.2 kg/m 3, so.
from solartribune.com
That means that water has a higher heat capacity—it can store more heat before changing in temperature. Air has a heat capacity of about 1000 joules per kg per °k and a density of just 1.2 kg/m 3, so. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). The cue ball rapidly decelerates (is cooled) as the. Water’s high heat capacity is a property caused by hydrogen bonding among water molecules. When heat is absorbed, hydrogen bonds are broken. A fast moving molecule (the cue ball) strikes a lattice of slow moving ice or water molecules. It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release thermal energy. Because of this, it takes more energy to heat water than it does other substances.
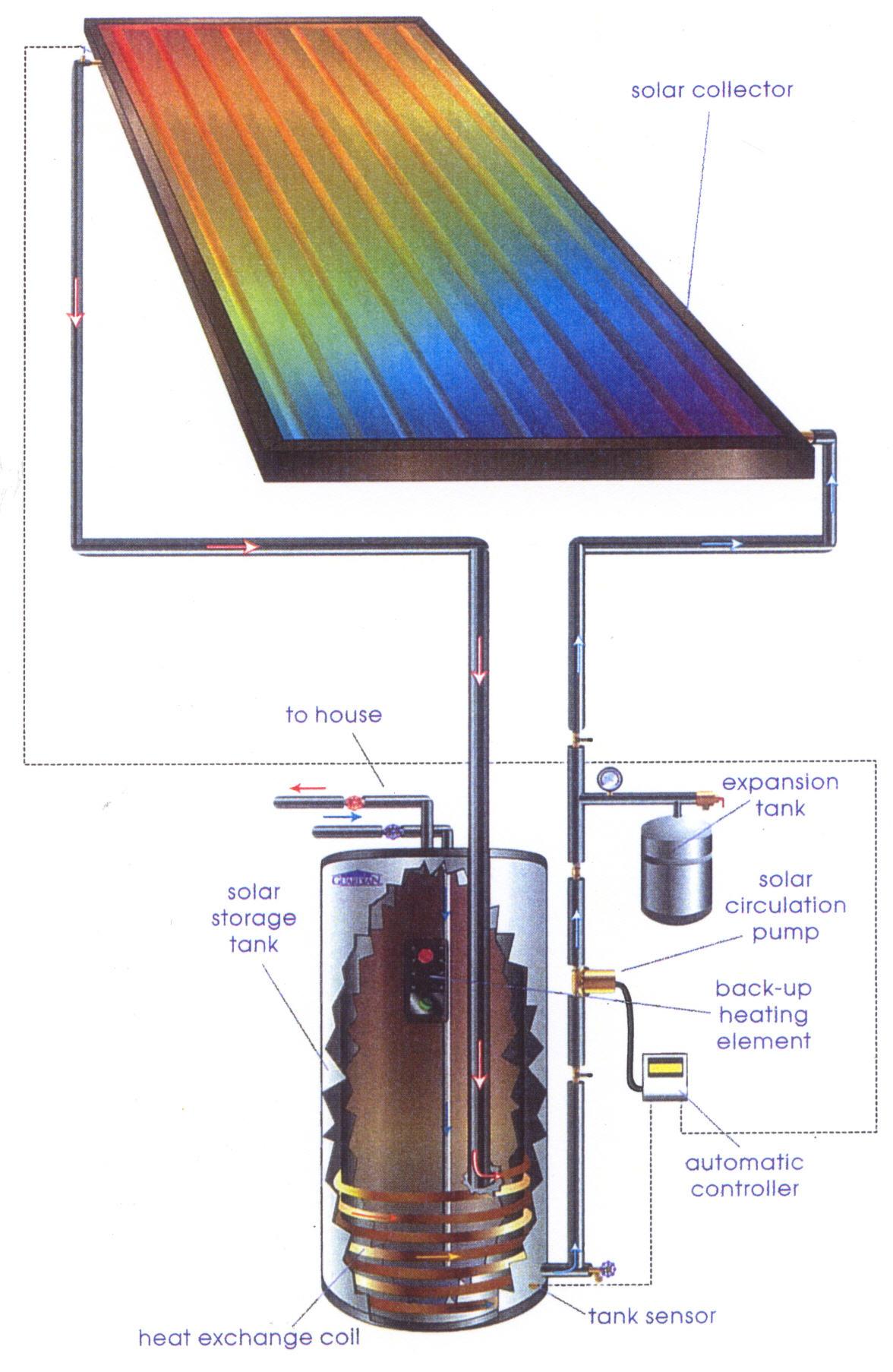
exchange diagram — Solar Tribune
Can Water Store More Heat Than Air Water’s high heat capacity is a property caused by hydrogen bonding among water molecules. Water’s high heat capacity is a property caused by hydrogen bonding among water molecules. Air has a heat capacity of about 1000 joules per kg per °k and a density of just 1.2 kg/m 3, so. It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release thermal energy. Because of this, it takes more energy to heat water than it does other substances. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). When heat is absorbed, hydrogen bonds are broken. That means that water has a higher heat capacity—it can store more heat before changing in temperature. The cue ball rapidly decelerates (is cooled) as the. A fast moving molecule (the cue ball) strikes a lattice of slow moving ice or water molecules.
From www.atkinson-builders.co.uk
Ground Source Heat Pumps Renewable Energy Atkinson Plumbing and Heating Can Water Store More Heat Than Air Because of this, it takes more energy to heat water than it does other substances. When heat is absorbed, hydrogen bonds are broken. A fast moving molecule (the cue ball) strikes a lattice of slow moving ice or water molecules. That means that water has a higher heat capacity—it can store more heat before changing in temperature. It plays a. Can Water Store More Heat Than Air.
From makinghouseswork.cchrc.org
What is a ground source heat pump? Making Houses Work Can Water Store More Heat Than Air The cue ball rapidly decelerates (is cooled) as the. Air has a heat capacity of about 1000 joules per kg per °k and a density of just 1.2 kg/m 3, so. That means that water has a higher heat capacity—it can store more heat before changing in temperature. Because of this, it takes more energy to heat water than it. Can Water Store More Heat Than Air.
From www.onecommunityglobal.org
Sustainable Water Heating Tank vs Tankless vs Heat Pumps in Offgrid Can Water Store More Heat Than Air It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release thermal energy. A fast moving molecule (the cue ball) strikes a lattice of slow moving ice or water molecules. That means that water has a higher heat capacity—it can store more heat before changing in temperature. The. Can Water Store More Heat Than Air.
From www.airandwater.com.au
How Does a Heat Pump Work? Air and Water Can Water Store More Heat Than Air It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release thermal energy. The cue ball rapidly decelerates (is cooled) as the. That means that water has a higher heat capacity—it can store more heat before changing in temperature. When heat is absorbed, hydrogen bonds are broken. Specific. Can Water Store More Heat Than Air.
From sayproperty.co.uk
SAY Property SAY's Guide to Air Source Heat Pumps SAY Property Can Water Store More Heat Than Air Because of this, it takes more energy to heat water than it does other substances. The cue ball rapidly decelerates (is cooled) as the. Water’s high heat capacity is a property caused by hydrogen bonding among water molecules. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree. Can Water Store More Heat Than Air.
From www.phcppros.com
Designing Heat Pump Water Heating Systems phcppros Can Water Store More Heat Than Air A fast moving molecule (the cue ball) strikes a lattice of slow moving ice or water molecules. The cue ball rapidly decelerates (is cooled) as the. Because of this, it takes more energy to heat water than it does other substances. Air has a heat capacity of about 1000 joules per kg per °k and a density of just 1.2. Can Water Store More Heat Than Air.
From civil-engineering-calculators.com
Solar Hot Water heater System Calculator, choose right water heater Can Water Store More Heat Than Air A fast moving molecule (the cue ball) strikes a lattice of slow moving ice or water molecules. Air has a heat capacity of about 1000 joules per kg per °k and a density of just 1.2 kg/m 3, so. When heat is absorbed, hydrogen bonds are broken. It plays a crucial role in understanding how different materials respond to heating. Can Water Store More Heat Than Air.
From pjharchitecturalservices.co.uk
PJH ECO SERIES AIR SOURCE HEAT PUMP RETROFIT PJH Architectural Services Can Water Store More Heat Than Air Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Because of this, it takes more energy to heat water than it does other substances. The cue ball rapidly decelerates (is cooled) as the. A fast moving molecule (the cue ball) strikes a lattice of slow. Can Water Store More Heat Than Air.
From www.infinity-energy.co.uk
Air Source Heat Pump Installers Infinity Energy Services Can Water Store More Heat Than Air Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Because of this, it takes more energy to heat water than it does other substances. When heat is absorbed, hydrogen bonds are broken. A fast moving molecule (the cue ball) strikes a lattice of slow moving. Can Water Store More Heat Than Air.
From www.aheatpump.com
3.5KW 300L Hot Water Storage tank All In One Water Heater Heat Pump Can Water Store More Heat Than Air Air has a heat capacity of about 1000 joules per kg per °k and a density of just 1.2 kg/m 3, so. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). When heat is absorbed, hydrogen bonds are broken. The cue ball rapidly decelerates (is. Can Water Store More Heat Than Air.
From www.remodeling.hw.net
Rheem Integrated HVAC and Water Heating System powered by Tankless Can Water Store More Heat Than Air Air has a heat capacity of about 1000 joules per kg per °k and a density of just 1.2 kg/m 3, so. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). A fast moving molecule (the cue ball) strikes a lattice of slow moving ice. Can Water Store More Heat Than Air.
From great-home.co.uk
typicalenergyuseheatpumpversusgasheatingt012 Great Home Can Water Store More Heat Than Air Water’s high heat capacity is a property caused by hydrogen bonding among water molecules. It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release thermal energy. Air has a heat capacity of about 1000 joules per kg per °k and a density of just 1.2 kg/m 3,. Can Water Store More Heat Than Air.
From www.cibsejournal.com
Module 205 Bivalent heat pump systems for heating and hot water Can Water Store More Heat Than Air Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). A fast moving molecule (the cue ball) strikes a lattice of slow moving ice or water molecules. Air has a heat capacity of about 1000 joules per kg per °k and a density of just 1.2. Can Water Store More Heat Than Air.
From sovremennaiaevropaimmediately.blogspot.com
do apartments share water heaters sovremennaiaevropaimmediately Can Water Store More Heat Than Air Because of this, it takes more energy to heat water than it does other substances. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store. Can Water Store More Heat Than Air.
From bestofwaterheater.blogspot.com
Storing Items Near Electric Water Heater Can Water Store More Heat Than Air Because of this, it takes more energy to heat water than it does other substances. When heat is absorbed, hydrogen bonds are broken. The cue ball rapidly decelerates (is cooled) as the. Water’s high heat capacity is a property caused by hydrogen bonding among water molecules. Specific heat is defined by the amount of heat needed to raise the temperature. Can Water Store More Heat Than Air.
From solartribune.com
exchange diagram — Solar Tribune Can Water Store More Heat Than Air When heat is absorbed, hydrogen bonds are broken. Water’s high heat capacity is a property caused by hydrogen bonding among water molecules. Air has a heat capacity of about 1000 joules per kg per °k and a density of just 1.2 kg/m 3, so. That means that water has a higher heat capacity—it can store more heat before changing in. Can Water Store More Heat Than Air.
From buildapullupbardarashin.blogspot.com
Build A Pull Up Bar Hot Water Heat Exchanger Can Water Store More Heat Than Air Because of this, it takes more energy to heat water than it does other substances. A fast moving molecule (the cue ball) strikes a lattice of slow moving ice or water molecules. That means that water has a higher heat capacity—it can store more heat before changing in temperature. When heat is absorbed, hydrogen bonds are broken. Specific heat is. Can Water Store More Heat Than Air.
From www.viridiansolar.co.uk
The different forms of solar energy Can Water Store More Heat Than Air Air has a heat capacity of about 1000 joules per kg per °k and a density of just 1.2 kg/m 3, so. A fast moving molecule (the cue ball) strikes a lattice of slow moving ice or water molecules. Water’s high heat capacity is a property caused by hydrogen bonding among water molecules. When heat is absorbed, hydrogen bonds are. Can Water Store More Heat Than Air.
From jmpcoblog.com
Introduction to Water Source Heat Pump Systems Part 3 Basic Operation Can Water Store More Heat Than Air Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Air has a heat capacity of about 1000 joules per kg per °k and a density of just 1.2 kg/m 3, so. Water’s high heat capacity is a property caused by hydrogen bonding among water molecules.. Can Water Store More Heat Than Air.
From gharpedia.com
Best Water HeaterHow to Choose as per the Need of Your Home? Can Water Store More Heat Than Air A fast moving molecule (the cue ball) strikes a lattice of slow moving ice or water molecules. Air has a heat capacity of about 1000 joules per kg per °k and a density of just 1.2 kg/m 3, so. Water’s high heat capacity is a property caused by hydrogen bonding among water molecules. Because of this, it takes more energy. Can Water Store More Heat Than Air.
From powerlines.seattle.gov
Why a heat pump water heater works for your home Powerlines Can Water Store More Heat Than Air Because of this, it takes more energy to heat water than it does other substances. It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release thermal energy. When heat is absorbed, hydrogen bonds are broken. Water’s high heat capacity is a property caused by hydrogen bonding among. Can Water Store More Heat Than Air.
From www.hydrosol.com.au
Heat pump hydronic heating can be powered by solar panels, cooling also Can Water Store More Heat Than Air A fast moving molecule (the cue ball) strikes a lattice of slow moving ice or water molecules. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). When heat is absorbed, hydrogen bonds are broken. That means that water has a higher heat capacity—it can store. Can Water Store More Heat Than Air.
From www.researchgate.net
Standard solar water heating system. Download Scientific Diagram Can Water Store More Heat Than Air A fast moving molecule (the cue ball) strikes a lattice of slow moving ice or water molecules. Air has a heat capacity of about 1000 joules per kg per °k and a density of just 1.2 kg/m 3, so. That means that water has a higher heat capacity—it can store more heat before changing in temperature. When heat is absorbed,. Can Water Store More Heat Than Air.
From www.winkelhage.com
Water Systems Mr. Winkelhage's site Can Water Store More Heat Than Air Water’s high heat capacity is a property caused by hydrogen bonding among water molecules. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). The cue ball rapidly decelerates (is cooled) as the. Because of this, it takes more energy to heat water than it does. Can Water Store More Heat Than Air.
From missehonorsbio.blogspot.com
EC Honors Biology Specific Heat and Water as a Solvent Can Water Store More Heat Than Air Water’s high heat capacity is a property caused by hydrogen bonding among water molecules. A fast moving molecule (the cue ball) strikes a lattice of slow moving ice or water molecules. Because of this, it takes more energy to heat water than it does other substances. Specific heat is defined by the amount of heat needed to raise the temperature. Can Water Store More Heat Than Air.
From australianenergyupgrades.com.au
Free hot water systems Australian Energy Upgrades Can Water Store More Heat Than Air The cue ball rapidly decelerates (is cooled) as the. Because of this, it takes more energy to heat water than it does other substances. Air has a heat capacity of about 1000 joules per kg per °k and a density of just 1.2 kg/m 3, so. That means that water has a higher heat capacity—it can store more heat before. Can Water Store More Heat Than Air.
From www.pinterest.com.mx
DPS MultiFuel Heat Bank Thermal Store Heating systems, Home heating Can Water Store More Heat Than Air Water’s high heat capacity is a property caused by hydrogen bonding among water molecules. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). The cue ball rapidly decelerates (is cooled) as the. It plays a crucial role in understanding how different materials respond to heating. Can Water Store More Heat Than Air.
From www.viridiansolar.co.uk
Solar Space Heating Can Water Store More Heat Than Air A fast moving molecule (the cue ball) strikes a lattice of slow moving ice or water molecules. Because of this, it takes more energy to heat water than it does other substances. That means that water has a higher heat capacity—it can store more heat before changing in temperature. Air has a heat capacity of about 1000 joules per kg. Can Water Store More Heat Than Air.
From ases.org
Passive Solar Heating American Solar Energy Society Can Water Store More Heat Than Air That means that water has a higher heat capacity—it can store more heat before changing in temperature. It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release thermal energy. Because of this, it takes more energy to heat water than it does other substances. Air has a. Can Water Store More Heat Than Air.
From modernize.com
Guide to Comparing Tank and Tankless Water Heaters Can Water Store More Heat Than Air A fast moving molecule (the cue ball) strikes a lattice of slow moving ice or water molecules. The cue ball rapidly decelerates (is cooled) as the. Air has a heat capacity of about 1000 joules per kg per °k and a density of just 1.2 kg/m 3, so. Specific heat is defined by the amount of heat needed to raise. Can Water Store More Heat Than Air.
From bottlefirst.com
Is A Hot Water Bottle Better Than A Heating Pad? Comparison! Can Water Store More Heat Than Air When heat is absorbed, hydrogen bonds are broken. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). A fast moving molecule (the cue ball) strikes a lattice of slow moving ice or water molecules. Air has a heat capacity of about 1000 joules per kg. Can Water Store More Heat Than Air.
From mepacademy.com
Steam Heating System Basics MEP Academy Can Water Store More Heat Than Air That means that water has a higher heat capacity—it can store more heat before changing in temperature. The cue ball rapidly decelerates (is cooled) as the. A fast moving molecule (the cue ball) strikes a lattice of slow moving ice or water molecules. It plays a crucial role in understanding how different materials respond to heating and cooling and describes. Can Water Store More Heat Than Air.
From firstechservices.com
HTP STORAGE TANK WATER HEATER Firstech Services Can Water Store More Heat Than Air Because of this, it takes more energy to heat water than it does other substances. The cue ball rapidly decelerates (is cooled) as the. A fast moving molecule (the cue ball) strikes a lattice of slow moving ice or water molecules. Air has a heat capacity of about 1000 joules per kg per °k and a density of just 1.2. Can Water Store More Heat Than Air.
From ressolar.com
Commercial Solar Hot Water Can Water Store More Heat Than Air The cue ball rapidly decelerates (is cooled) as the. That means that water has a higher heat capacity—it can store more heat before changing in temperature. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Because of this, it takes more energy to heat water. Can Water Store More Heat Than Air.
From bottlefirst.com
Is A Hot Water Bottle Better Than A Heating Pad? Comparison! Can Water Store More Heat Than Air It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release thermal energy. The cue ball rapidly decelerates (is cooled) as the. Because of this, it takes more energy to heat water than it does other substances. When heat is absorbed, hydrogen bonds are broken. Specific heat is. Can Water Store More Heat Than Air.