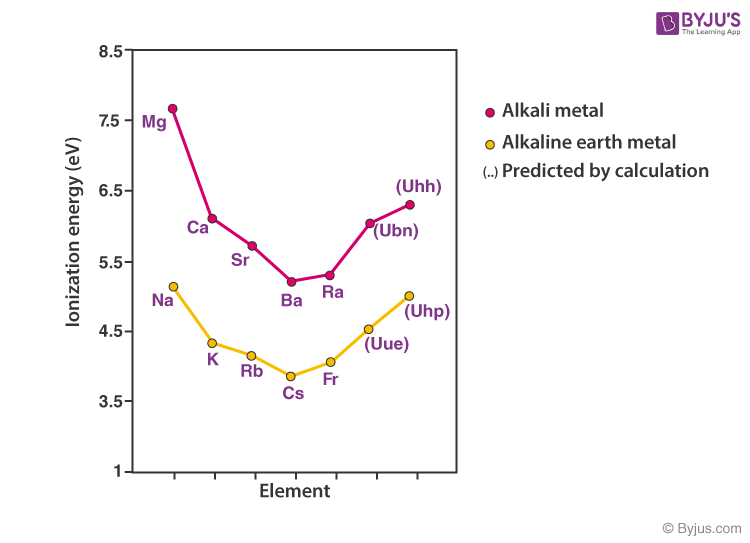
Alkali Metal Group Properties . The alkali metals are lithium, sodium, potassium, rubidium,. the group 1 elements are all soft, reactive metals with low melting points. This lone electron is loosely bound, making this a set of reactive metallic elements. — as a group, the alkali metals have a looser crystallographic arrangement than any of the other metallic crystals,. — the alkali metals are the elements located in group ia of the periodic table (the first column). — the alkali metals are the elements located in group ia of the periodic table. They react with water to produce an alkaline metal hydroxide solution and hydrogen. The key characteristic these elements share in common is that they all have one electron in the outer electron shell. the variations in ionization energy and solvation energies down the alkali metal group help explain why li is more reducing than the other alkali metals. Reactivity increases down the group.
from byjus.com
The alkali metals are lithium, sodium, potassium, rubidium,. This lone electron is loosely bound, making this a set of reactive metallic elements. the group 1 elements are all soft, reactive metals with low melting points. The key characteristic these elements share in common is that they all have one electron in the outer electron shell. — the alkali metals are the elements located in group ia of the periodic table. the variations in ionization energy and solvation energies down the alkali metal group help explain why li is more reducing than the other alkali metals. Reactivity increases down the group. — as a group, the alkali metals have a looser crystallographic arrangement than any of the other metallic crystals,. They react with water to produce an alkaline metal hydroxide solution and hydrogen. — the alkali metals are the elements located in group ia of the periodic table (the first column).
Alkali Metals Properties, Electronic Configuration, Periodic Trends
Alkali Metal Group Properties Reactivity increases down the group. The key characteristic these elements share in common is that they all have one electron in the outer electron shell. Reactivity increases down the group. This lone electron is loosely bound, making this a set of reactive metallic elements. the variations in ionization energy and solvation energies down the alkali metal group help explain why li is more reducing than the other alkali metals. the group 1 elements are all soft, reactive metals with low melting points. — as a group, the alkali metals have a looser crystallographic arrangement than any of the other metallic crystals,. — the alkali metals are the elements located in group ia of the periodic table. They react with water to produce an alkaline metal hydroxide solution and hydrogen. — the alkali metals are the elements located in group ia of the periodic table (the first column). The alkali metals are lithium, sodium, potassium, rubidium,.
From www.slideserve.com
PPT Group 1 The alkali metals PowerPoint Presentation ID5525387 Alkali Metal Group Properties — the alkali metals are the elements located in group ia of the periodic table. Reactivity increases down the group. This lone electron is loosely bound, making this a set of reactive metallic elements. The key characteristic these elements share in common is that they all have one electron in the outer electron shell. the variations in ionization. Alkali Metal Group Properties.
From awesomehome.co
Alkali Metals Periodic Table Located Awesome Home Alkali Metal Group Properties The key characteristic these elements share in common is that they all have one electron in the outer electron shell. — the alkali metals are the elements located in group ia of the periodic table (the first column). the variations in ionization energy and solvation energies down the alkali metal group help explain why li is more reducing. Alkali Metal Group Properties.
From studymind.co.uk
The Transition Metals (GCSE Chemistry) Study Mind Alkali Metal Group Properties — the alkali metals are the elements located in group ia of the periodic table (the first column). — as a group, the alkali metals have a looser crystallographic arrangement than any of the other metallic crystals,. Reactivity increases down the group. They react with water to produce an alkaline metal hydroxide solution and hydrogen. This lone electron. Alkali Metal Group Properties.
From overallscience.com
Trends in atomic and physical properties of alkali metals Overall Science Alkali Metal Group Properties This lone electron is loosely bound, making this a set of reactive metallic elements. The key characteristic these elements share in common is that they all have one electron in the outer electron shell. — the alkali metals are the elements located in group ia of the periodic table (the first column). the group 1 elements are all. Alkali Metal Group Properties.
From selfstudypoint.in
Group 1 Elements Alkali Metals Alkali Metal Group Properties — as a group, the alkali metals have a looser crystallographic arrangement than any of the other metallic crystals,. The alkali metals are lithium, sodium, potassium, rubidium,. — the alkali metals are the elements located in group ia of the periodic table (the first column). They react with water to produce an alkaline metal hydroxide solution and hydrogen.. Alkali Metal Group Properties.
From elchoroukhost.net
Alkali Metals Periodic Table Location Elcho Table Alkali Metal Group Properties The key characteristic these elements share in common is that they all have one electron in the outer electron shell. Reactivity increases down the group. — the alkali metals are the elements located in group ia of the periodic table. the group 1 elements are all soft, reactive metals with low melting points. — the alkali metals. Alkali Metal Group Properties.
From saylordotorg.github.io
The Alkali Metals (Group 1) Alkali Metal Group Properties — as a group, the alkali metals have a looser crystallographic arrangement than any of the other metallic crystals,. The alkali metals are lithium, sodium, potassium, rubidium,. They react with water to produce an alkaline metal hydroxide solution and hydrogen. — the alkali metals are the elements located in group ia of the periodic table (the first column).. Alkali Metal Group Properties.
From www.chemistry4students.com
Chemistry 4 Students Alkali Metals (group 1 elements) Alkali Metal Group Properties Reactivity increases down the group. the variations in ionization energy and solvation energies down the alkali metal group help explain why li is more reducing than the other alkali metals. The key characteristic these elements share in common is that they all have one electron in the outer electron shell. The alkali metals are lithium, sodium, potassium, rubidium,. . Alkali Metal Group Properties.
From www.breakingatom.com
Group 1 The Alkali Metals Alkali Metal Group Properties — the alkali metals are the elements located in group ia of the periodic table. The alkali metals are lithium, sodium, potassium, rubidium,. This lone electron is loosely bound, making this a set of reactive metallic elements. The key characteristic these elements share in common is that they all have one electron in the outer electron shell. the. Alkali Metal Group Properties.
From spmchemistry.blog.onlinetuition.com.my
Physical Properties of Alkali Metals SPM Chemistry Alkali Metal Group Properties — as a group, the alkali metals have a looser crystallographic arrangement than any of the other metallic crystals,. the group 1 elements are all soft, reactive metals with low melting points. This lone electron is loosely bound, making this a set of reactive metallic elements. — the alkali metals are the elements located in group ia. Alkali Metal Group Properties.
From ravennewsrogers.blogspot.com
Describe the Properties of Alkali Metals Alkali Metal Group Properties — the alkali metals are the elements located in group ia of the periodic table (the first column). — the alkali metals are the elements located in group ia of the periodic table. They react with water to produce an alkaline metal hydroxide solution and hydrogen. This lone electron is loosely bound, making this a set of reactive. Alkali Metal Group Properties.
From m20131000606.blogspot.com
Chemistry Group 1 Elements Alkali Metals Alkali Metal Group Properties Reactivity increases down the group. the group 1 elements are all soft, reactive metals with low melting points. — as a group, the alkali metals have a looser crystallographic arrangement than any of the other metallic crystals,. — the alkali metals are the elements located in group ia of the periodic table. — the alkali metals. Alkali Metal Group Properties.
From sciencenotes.org
Alkali Metal Properties Alkali Metal Group Properties the variations in ionization energy and solvation energies down the alkali metal group help explain why li is more reducing than the other alkali metals. — the alkali metals are the elements located in group ia of the periodic table (the first column). — as a group, the alkali metals have a looser crystallographic arrangement than any. Alkali Metal Group Properties.
From www.tes.com
The Alkali Metals Group 1 Teaching Resources Alkali Metal Group Properties the group 1 elements are all soft, reactive metals with low melting points. — as a group, the alkali metals have a looser crystallographic arrangement than any of the other metallic crystals,. Reactivity increases down the group. They react with water to produce an alkaline metal hydroxide solution and hydrogen. The alkali metals are lithium, sodium, potassium, rubidium,.. Alkali Metal Group Properties.
From byjus.com
Alkali Metals Properties, Electronic Configuration, Periodic Trends Alkali Metal Group Properties The alkali metals are lithium, sodium, potassium, rubidium,. The key characteristic these elements share in common is that they all have one electron in the outer electron shell. — the alkali metals are the elements located in group ia of the periodic table (the first column). the group 1 elements are all soft, reactive metals with low melting. Alkali Metal Group Properties.
From exobgridh.blob.core.windows.net
What Are The Common Physical Properties Of Alkali Metals at Israel Alkali Metal Group Properties This lone electron is loosely bound, making this a set of reactive metallic elements. Reactivity increases down the group. — as a group, the alkali metals have a looser crystallographic arrangement than any of the other metallic crystals,. The alkali metals are lithium, sodium, potassium, rubidium,. — the alkali metals are the elements located in group ia of. Alkali Metal Group Properties.
From www.tes.com
Lesson Alkali Metals GCSE Edexcel 91 Teaching Resources Alkali Metal Group Properties — as a group, the alkali metals have a looser crystallographic arrangement than any of the other metallic crystals,. the group 1 elements are all soft, reactive metals with low melting points. This lone electron is loosely bound, making this a set of reactive metallic elements. Reactivity increases down the group. — the alkali metals are the. Alkali Metal Group Properties.
From www.nagwa.com
Question Video Identifying the Property of Alkali Metals From a List Alkali Metal Group Properties — the alkali metals are the elements located in group ia of the periodic table. The key characteristic these elements share in common is that they all have one electron in the outer electron shell. They react with water to produce an alkaline metal hydroxide solution and hydrogen. The alkali metals are lithium, sodium, potassium, rubidium,. Reactivity increases down. Alkali Metal Group Properties.
From www.slideserve.com
PPT The Alkali Metals PowerPoint Presentation, free download ID2927807 Alkali Metal Group Properties The alkali metals are lithium, sodium, potassium, rubidium,. The key characteristic these elements share in common is that they all have one electron in the outer electron shell. Reactivity increases down the group. the group 1 elements are all soft, reactive metals with low melting points. the variations in ionization energy and solvation energies down the alkali metal. Alkali Metal Group Properties.
From thechemistrynotes.com
Trends of the Properties of Group 2 (Alkaline Earth) Metals Alkali Metal Group Properties — as a group, the alkali metals have a looser crystallographic arrangement than any of the other metallic crystals,. the variations in ionization energy and solvation energies down the alkali metal group help explain why li is more reducing than the other alkali metals. — the alkali metals are the elements located in group ia of the. Alkali Metal Group Properties.
From online-learning-college.com
Group 1 alkali metals Properties of alkali metals Reactions Alkali Metal Group Properties — the alkali metals are the elements located in group ia of the periodic table (the first column). They react with water to produce an alkaline metal hydroxide solution and hydrogen. The key characteristic these elements share in common is that they all have one electron in the outer electron shell. This lone electron is loosely bound, making this. Alkali Metal Group Properties.
From elchoroukhost.net
Alkali Metals Periodic Table Properties Elcho Table Alkali Metal Group Properties — the alkali metals are the elements located in group ia of the periodic table. the group 1 elements are all soft, reactive metals with low melting points. They react with water to produce an alkaline metal hydroxide solution and hydrogen. The alkali metals are lithium, sodium, potassium, rubidium,. Reactivity increases down the group. — the alkali. Alkali Metal Group Properties.
From awesomehome.co
Alkali Metals Periodic Table Awesome Home Alkali Metal Group Properties the variations in ionization energy and solvation energies down the alkali metal group help explain why li is more reducing than the other alkali metals. — the alkali metals are the elements located in group ia of the periodic table. They react with water to produce an alkaline metal hydroxide solution and hydrogen. This lone electron is loosely. Alkali Metal Group Properties.
From xlskoor.blogspot.com
Alkali Metals Chemistry Alkali Metal Group Properties The alkali metals are lithium, sodium, potassium, rubidium,. — as a group, the alkali metals have a looser crystallographic arrangement than any of the other metallic crystals,. — the alkali metals are the elements located in group ia of the periodic table. The key characteristic these elements share in common is that they all have one electron in. Alkali Metal Group Properties.
From www.slideserve.com
PPT THE ALKALI METALS PowerPoint Presentation, free download ID2323748 Alkali Metal Group Properties Reactivity increases down the group. The alkali metals are lithium, sodium, potassium, rubidium,. This lone electron is loosely bound, making this a set of reactive metallic elements. They react with water to produce an alkaline metal hydroxide solution and hydrogen. — the alkali metals are the elements located in group ia of the periodic table. — as a. Alkali Metal Group Properties.
From elchoroukhost.net
Properties Of Alkali Metals On The Periodic Table Elcho Table Alkali Metal Group Properties — as a group, the alkali metals have a looser crystallographic arrangement than any of the other metallic crystals,. the group 1 elements are all soft, reactive metals with low melting points. The key characteristic these elements share in common is that they all have one electron in the outer electron shell. They react with water to produce. Alkali Metal Group Properties.
From elchoroukhost.net
Alkali Metals Periodic Table Reactivity Elcho Table Alkali Metal Group Properties — the alkali metals are the elements located in group ia of the periodic table. The key characteristic these elements share in common is that they all have one electron in the outer electron shell. the variations in ionization energy and solvation energies down the alkali metal group help explain why li is more reducing than the other. Alkali Metal Group Properties.
From www.slideserve.com
PPT Introduction to the Periodic Table of Elements PowerPoint Alkali Metal Group Properties The key characteristic these elements share in common is that they all have one electron in the outer electron shell. the variations in ionization energy and solvation energies down the alkali metal group help explain why li is more reducing than the other alkali metals. This lone electron is loosely bound, making this a set of reactive metallic elements.. Alkali Metal Group Properties.
From www.pinterest.co.uk
Group 1 (Alkali Metals) Alkali metal, Physical properties, Physics Alkali Metal Group Properties The alkali metals are lithium, sodium, potassium, rubidium,. This lone electron is loosely bound, making this a set of reactive metallic elements. The key characteristic these elements share in common is that they all have one electron in the outer electron shell. Reactivity increases down the group. the variations in ionization energy and solvation energies down the alkali metal. Alkali Metal Group Properties.
From www.britannica.com
alkali metal Definition, Properties, & Facts Britannica Alkali Metal Group Properties They react with water to produce an alkaline metal hydroxide solution and hydrogen. The key characteristic these elements share in common is that they all have one electron in the outer electron shell. the variations in ionization energy and solvation energies down the alkali metal group help explain why li is more reducing than the other alkali metals. . Alkali Metal Group Properties.
From exobgridh.blob.core.windows.net
What Are The Common Physical Properties Of Alkali Metals at Israel Alkali Metal Group Properties The alkali metals are lithium, sodium, potassium, rubidium,. the group 1 elements are all soft, reactive metals with low melting points. Reactivity increases down the group. This lone electron is loosely bound, making this a set of reactive metallic elements. The key characteristic these elements share in common is that they all have one electron in the outer electron. Alkali Metal Group Properties.
From online-learning-college.com
Group 1 alkali metals Properties of alkali metals Reactions Alkali Metal Group Properties — as a group, the alkali metals have a looser crystallographic arrangement than any of the other metallic crystals,. They react with water to produce an alkaline metal hydroxide solution and hydrogen. This lone electron is loosely bound, making this a set of reactive metallic elements. The key characteristic these elements share in common is that they all have. Alkali Metal Group Properties.
From byjus.com
Alkali Metals Chemical and Physical Properties of Alkali Metals Alkali Metal Group Properties — as a group, the alkali metals have a looser crystallographic arrangement than any of the other metallic crystals,. They react with water to produce an alkaline metal hydroxide solution and hydrogen. The key characteristic these elements share in common is that they all have one electron in the outer electron shell. the group 1 elements are all. Alkali Metal Group Properties.
From www.vedantu.com
Alkali Metals Chemical Elements, Properties Alkali Metals Periodic Alkali Metal Group Properties This lone electron is loosely bound, making this a set of reactive metallic elements. — as a group, the alkali metals have a looser crystallographic arrangement than any of the other metallic crystals,. They react with water to produce an alkaline metal hydroxide solution and hydrogen. the group 1 elements are all soft, reactive metals with low melting. Alkali Metal Group Properties.
From spmchemistry.blog.onlinetuition.com.my
Physical Properties of Alkali Metals SPM Chemistry Alkali Metal Group Properties the variations in ionization energy and solvation energies down the alkali metal group help explain why li is more reducing than the other alkali metals. Reactivity increases down the group. This lone electron is loosely bound, making this a set of reactive metallic elements. — the alkali metals are the elements located in group ia of the periodic. Alkali Metal Group Properties.