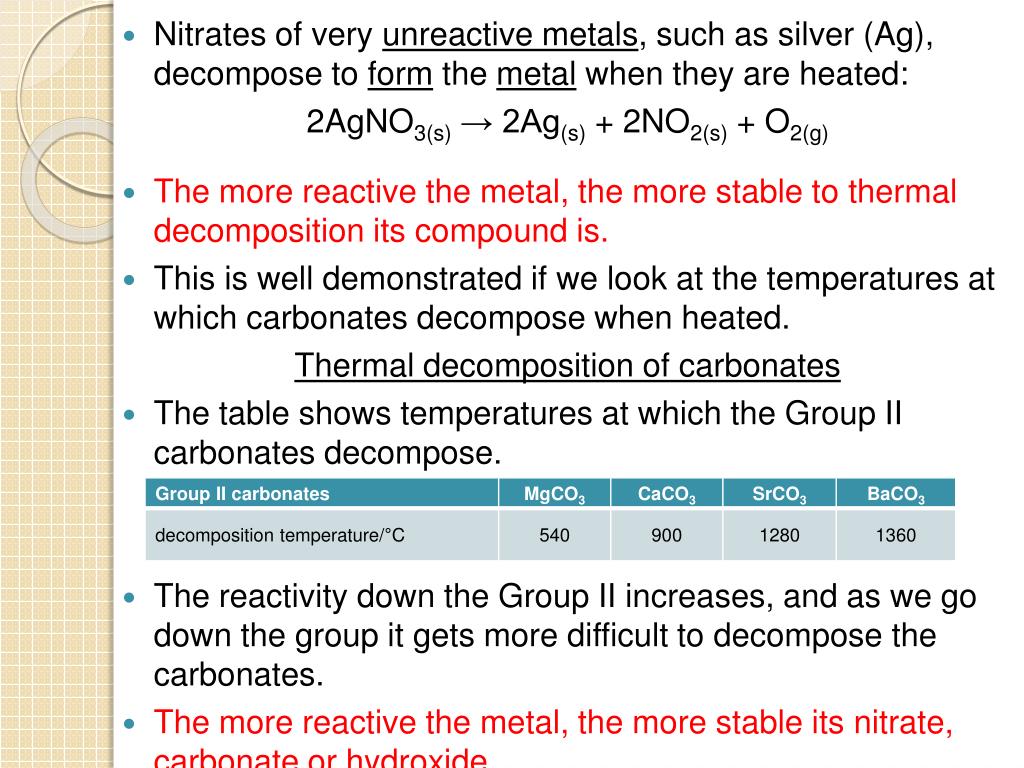
Zinc Hydroxide Thermal Decomposition Temperature . Thermal decomposition of metal carbonates. Metal carbonates decompose when heated. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition. — the effects of calcination temperatures, i.e., 300, 500, 650, 700, and 750 °c, on the crystallinity, optical. — results showed that zinc carbonate hydroxide decomposition started at about 150°c and the rate of decomposition became significant at. In association with nuffield foundation. — this study is devoted to the thermal decomposition of two zinc carbonate hydroxide samples up to 400 °c.
from www.slideserve.com
— the effects of calcination temperatures, i.e., 300, 500, 650, 700, and 750 °c, on the crystallinity, optical. — this study is devoted to the thermal decomposition of two zinc carbonate hydroxide samples up to 400 °c. results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition. Thermal decomposition of metal carbonates. — results showed that zinc carbonate hydroxide decomposition started at about 150°c and the rate of decomposition became significant at. In association with nuffield foundation. Metal carbonates decompose when heated. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper.
PPT Thermal PowerPoint Presentation, free download ID
Zinc Hydroxide Thermal Decomposition Temperature — the effects of calcination temperatures, i.e., 300, 500, 650, 700, and 750 °c, on the crystallinity, optical. In association with nuffield foundation. — results showed that zinc carbonate hydroxide decomposition started at about 150°c and the rate of decomposition became significant at. Thermal decomposition of metal carbonates. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. Metal carbonates decompose when heated. results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition. — the effects of calcination temperatures, i.e., 300, 500, 650, 700, and 750 °c, on the crystallinity, optical. — this study is devoted to the thermal decomposition of two zinc carbonate hydroxide samples up to 400 °c.
From www.researchgate.net
(PDF) Thermal of zinc hydroxysulfatehydrate minerals Zinc Hydroxide Thermal Decomposition Temperature Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. Thermal decomposition of metal carbonates. Metal carbonates decompose when heated. In association with nuffield foundation. — the effects of calcination temperatures, i.e., 300, 500, 650, 700, and 750 °c, on the crystallinity, optical. —. Zinc Hydroxide Thermal Decomposition Temperature.
From www.researchgate.net
Powder Xray diffraction patterns of zinc hydroxy... Download Zinc Hydroxide Thermal Decomposition Temperature Metal carbonates decompose when heated. — the effects of calcination temperatures, i.e., 300, 500, 650, 700, and 750 °c, on the crystallinity, optical. In association with nuffield foundation. results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition. — results showed that zinc carbonate hydroxide decomposition started at about 150°c. Zinc Hydroxide Thermal Decomposition Temperature.
From www.semanticscholar.org
[PDF] Thermal of zinc carbonate hydroxide Semantic Scholar Zinc Hydroxide Thermal Decomposition Temperature In association with nuffield foundation. results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition. — the effects of calcination temperatures, i.e., 300, 500, 650, 700, and 750 °c, on the crystallinity, optical. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less. Zinc Hydroxide Thermal Decomposition Temperature.
From www.youtube.com
Thermal of Zinc Carbonate YouTube Zinc Hydroxide Thermal Decomposition Temperature results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition. Thermal decomposition of metal carbonates. In association with nuffield foundation. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. — the effects of calcination temperatures, i.e.,. Zinc Hydroxide Thermal Decomposition Temperature.
From www.researchgate.net
Thermal data of the compounds Download Table Zinc Hydroxide Thermal Decomposition Temperature — this study is devoted to the thermal decomposition of two zinc carbonate hydroxide samples up to 400 °c. — the effects of calcination temperatures, i.e., 300, 500, 650, 700, and 750 °c, on the crystallinity, optical. — results showed that zinc carbonate hydroxide decomposition started at about 150°c and the rate of decomposition became significant at.. Zinc Hydroxide Thermal Decomposition Temperature.
From www.semanticscholar.org
[PDF] Thermal of zinc carbonate hydroxide Semantic Scholar Zinc Hydroxide Thermal Decomposition Temperature Thermal decomposition of metal carbonates. Metal carbonates decompose when heated. results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. In association with nuffield foundation. — this. Zinc Hydroxide Thermal Decomposition Temperature.
From www.researchgate.net
Conversion degree versus temperature of thermal of Zinc Hydroxide Thermal Decomposition Temperature — this study is devoted to the thermal decomposition of two zinc carbonate hydroxide samples up to 400 °c. Metal carbonates decompose when heated. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. — results showed that zinc carbonate hydroxide decomposition started at. Zinc Hydroxide Thermal Decomposition Temperature.
From www.slideserve.com
PPT Thermal PowerPoint Presentation ID544610 Zinc Hydroxide Thermal Decomposition Temperature — this study is devoted to the thermal decomposition of two zinc carbonate hydroxide samples up to 400 °c. — the effects of calcination temperatures, i.e., 300, 500, 650, 700, and 750 °c, on the crystallinity, optical. Metal carbonates decompose when heated. results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate. Zinc Hydroxide Thermal Decomposition Temperature.
From www.youtube.com
How to Balance Zn(OH)2 = ZnO + H2O (Zinc hydroxide YouTube Zinc Hydroxide Thermal Decomposition Temperature — the effects of calcination temperatures, i.e., 300, 500, 650, 700, and 750 °c, on the crystallinity, optical. Thermal decomposition of metal carbonates. results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less. Zinc Hydroxide Thermal Decomposition Temperature.
From www.researchgate.net
Thermal profile of zinc hydroxide nitrate treated in (a Zinc Hydroxide Thermal Decomposition Temperature In association with nuffield foundation. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. Metal carbonates decompose when heated. — this study is devoted to the thermal decomposition of two zinc carbonate hydroxide samples up to 400 °c. — results showed that zinc. Zinc Hydroxide Thermal Decomposition Temperature.
From www.semanticscholar.org
[PDF] Thermal of zinc carbonate hydroxide Semantic Scholar Zinc Hydroxide Thermal Decomposition Temperature — this study is devoted to the thermal decomposition of two zinc carbonate hydroxide samples up to 400 °c. — results showed that zinc carbonate hydroxide decomposition started at about 150°c and the rate of decomposition became significant at. In association with nuffield foundation. — the effects of calcination temperatures, i.e., 300, 500, 650, 700, and 750. Zinc Hydroxide Thermal Decomposition Temperature.
From www.slideserve.com
PPT Thermal PowerPoint Presentation, free download ID Zinc Hydroxide Thermal Decomposition Temperature Metal carbonates decompose when heated. In association with nuffield foundation. Thermal decomposition of metal carbonates. results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition. — the effects of calcination temperatures, i.e., 300, 500, 650, 700, and 750 °c, on the crystallinity, optical. — results showed that zinc carbonate hydroxide. Zinc Hydroxide Thermal Decomposition Temperature.
From www.researchgate.net
Nonisothermal thermal curves of Ca(OH) 2 and selected Zinc Hydroxide Thermal Decomposition Temperature In association with nuffield foundation. — this study is devoted to the thermal decomposition of two zinc carbonate hydroxide samples up to 400 °c. — the effects of calcination temperatures, i.e., 300, 500, 650, 700, and 750 °c, on the crystallinity, optical. Thermal decomposition of metal carbonates. Compare the thermal stabilities of carbonates of reactive metals, like sodium. Zinc Hydroxide Thermal Decomposition Temperature.
From www.semanticscholar.org
[PDF] Thermal of zinc carbonate hydroxide Semantic Scholar Zinc Hydroxide Thermal Decomposition Temperature — this study is devoted to the thermal decomposition of two zinc carbonate hydroxide samples up to 400 °c. In association with nuffield foundation. — the effects of calcination temperatures, i.e., 300, 500, 650, 700, and 750 °c, on the crystallinity, optical. — results showed that zinc carbonate hydroxide decomposition started at about 150°c and the rate. Zinc Hydroxide Thermal Decomposition Temperature.
From www.researchgate.net
(PDF) Influences of evolved gases on the thermal of zinc Zinc Hydroxide Thermal Decomposition Temperature results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition. Metal carbonates decompose when heated. In association with nuffield foundation. — this study is devoted to the thermal decomposition of two zinc carbonate hydroxide samples up to 400 °c. Thermal decomposition of metal carbonates. — the effects of calcination temperatures,. Zinc Hydroxide Thermal Decomposition Temperature.
From www.semanticscholar.org
[PDF] Thermal of zinc carbonate hydroxide Semantic Scholar Zinc Hydroxide Thermal Decomposition Temperature — the effects of calcination temperatures, i.e., 300, 500, 650, 700, and 750 °c, on the crystallinity, optical. In association with nuffield foundation. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. — this study is devoted to the thermal decomposition of two. Zinc Hydroxide Thermal Decomposition Temperature.
From www.researchgate.net
TGDTG curve of thermal of zinc acetate dihydrate at Zinc Hydroxide Thermal Decomposition Temperature — this study is devoted to the thermal decomposition of two zinc carbonate hydroxide samples up to 400 °c. results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition. — results showed that zinc carbonate hydroxide decomposition started at about 150°c and the rate of decomposition became significant at. In. Zinc Hydroxide Thermal Decomposition Temperature.
From www.researchgate.net
Reaction scheme showing the thermal of zinc (II Zinc Hydroxide Thermal Decomposition Temperature — the effects of calcination temperatures, i.e., 300, 500, 650, 700, and 750 °c, on the crystallinity, optical. — results showed that zinc carbonate hydroxide decomposition started at about 150°c and the rate of decomposition became significant at. Metal carbonates decompose when heated. — this study is devoted to the thermal decomposition of two zinc carbonate hydroxide. Zinc Hydroxide Thermal Decomposition Temperature.
From www.researchgate.net
(PDF) Effect of CO2 partial pressure on the thermal Zinc Hydroxide Thermal Decomposition Temperature In association with nuffield foundation. — the effects of calcination temperatures, i.e., 300, 500, 650, 700, and 750 °c, on the crystallinity, optical. Metal carbonates decompose when heated. results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition. — results showed that zinc carbonate hydroxide decomposition started at about 150°c. Zinc Hydroxide Thermal Decomposition Temperature.
From www.youtube.com
Thermal of Zinc Carbonate YouTube Zinc Hydroxide Thermal Decomposition Temperature In association with nuffield foundation. — results showed that zinc carbonate hydroxide decomposition started at about 150°c and the rate of decomposition became significant at. — this study is devoted to the thermal decomposition of two zinc carbonate hydroxide samples up to 400 °c. Thermal decomposition of metal carbonates. Metal carbonates decompose when heated. results showed that. Zinc Hydroxide Thermal Decomposition Temperature.
From www.researchgate.net
Experimental and model results conversion values of the thermal Zinc Hydroxide Thermal Decomposition Temperature — this study is devoted to the thermal decomposition of two zinc carbonate hydroxide samples up to 400 °c. In association with nuffield foundation. Metal carbonates decompose when heated. — results showed that zinc carbonate hydroxide decomposition started at about 150°c and the rate of decomposition became significant at. results showed that zinc carbonate hydroxide decomposition started. Zinc Hydroxide Thermal Decomposition Temperature.
From www.slideserve.com
PPT Thermal Reactions PowerPoint Presentation, free Zinc Hydroxide Thermal Decomposition Temperature Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. Metal carbonates decompose when heated. Thermal decomposition of metal carbonates. In association with nuffield foundation. — this study is devoted to the thermal decomposition of two zinc carbonate hydroxide samples up to 400 °c. . Zinc Hydroxide Thermal Decomposition Temperature.
From www.nagwa.com
Question Video Identifying the Correct Chemical Equation for the Zinc Hydroxide Thermal Decomposition Temperature results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition. — this study is devoted to the thermal decomposition of two zinc carbonate hydroxide samples up to 400 °c. Thermal decomposition of metal carbonates. — results showed that zinc carbonate hydroxide decomposition started at about 150°c and the rate of. Zinc Hydroxide Thermal Decomposition Temperature.
From www.semanticscholar.org
Figure 4 from Effect of pH, concentration and temperature on copper and Zinc Hydroxide Thermal Decomposition Temperature Metal carbonates decompose when heated. — results showed that zinc carbonate hydroxide decomposition started at about 150°c and the rate of decomposition became significant at. Thermal decomposition of metal carbonates. results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition. — the effects of calcination temperatures, i.e., 300, 500, 650,. Zinc Hydroxide Thermal Decomposition Temperature.
From www.researchgate.net
(PDF) Effect of CO2 partial pressure on the thermal Zinc Hydroxide Thermal Decomposition Temperature In association with nuffield foundation. — this study is devoted to the thermal decomposition of two zinc carbonate hydroxide samples up to 400 °c. — results showed that zinc carbonate hydroxide decomposition started at about 150°c and the rate of decomposition became significant at. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and. Zinc Hydroxide Thermal Decomposition Temperature.
From www.researchgate.net
Thermal profile of zinc hydroxide nitrate treated in (a Zinc Hydroxide Thermal Decomposition Temperature Metal carbonates decompose when heated. — results showed that zinc carbonate hydroxide decomposition started at about 150°c and the rate of decomposition became significant at. Thermal decomposition of metal carbonates. In association with nuffield foundation. — the effects of calcination temperatures, i.e., 300, 500, 650, 700, and 750 °c, on the crystallinity, optical. results showed that zinc. Zinc Hydroxide Thermal Decomposition Temperature.
From www.semanticscholar.org
[PDF] Thermal of zinc carbonate hydroxide Semantic Scholar Zinc Hydroxide Thermal Decomposition Temperature Metal carbonates decompose when heated. — results showed that zinc carbonate hydroxide decomposition started at about 150°c and the rate of decomposition became significant at. — this study is devoted to the thermal decomposition of two zinc carbonate hydroxide samples up to 400 °c. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and. Zinc Hydroxide Thermal Decomposition Temperature.
From www.researchgate.net
TGADTA thermograms the thermal behavior of poly(vinyl Zinc Hydroxide Thermal Decomposition Temperature Metal carbonates decompose when heated. results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition. Thermal decomposition of metal carbonates. — results showed that zinc carbonate hydroxide decomposition started at about 150°c and the rate of decomposition became significant at. Compare the thermal stabilities of carbonates of reactive metals, like sodium. Zinc Hydroxide Thermal Decomposition Temperature.
From keystagewiki.com
Thermal Key Stage Wiki Zinc Hydroxide Thermal Decomposition Temperature — results showed that zinc carbonate hydroxide decomposition started at about 150°c and the rate of decomposition became significant at. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. In association with nuffield foundation. — this study is devoted to the thermal decomposition. Zinc Hydroxide Thermal Decomposition Temperature.
From www.researchgate.net
(PDF) Thermal of zinc carbonate hydroxide Zinc Hydroxide Thermal Decomposition Temperature — results showed that zinc carbonate hydroxide decomposition started at about 150°c and the rate of decomposition became significant at. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. — this study is devoted to the thermal decomposition of two zinc carbonate hydroxide. Zinc Hydroxide Thermal Decomposition Temperature.
From pubs.acs.org
Layered Zinc Hydroxide Dihydrate, Zn5(OH)10·2H2O, from Hydrothermal Zinc Hydroxide Thermal Decomposition Temperature — this study is devoted to the thermal decomposition of two zinc carbonate hydroxide samples up to 400 °c. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. — results showed that zinc carbonate hydroxide decomposition started at about 150°c and the rate. Zinc Hydroxide Thermal Decomposition Temperature.
From pubs.acs.org
Effect of Zinc Oxide on the Thermal of Dimethyl Sulfoxide Zinc Hydroxide Thermal Decomposition Temperature Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. Metal carbonates decompose when heated. Thermal decomposition of metal carbonates. In association with nuffield foundation. — this study is devoted to the thermal decomposition of two zinc carbonate hydroxide samples up to 400 °c. . Zinc Hydroxide Thermal Decomposition Temperature.
From www.researchgate.net
(PDF) Influences of evolved gases on the thermal of zinc Zinc Hydroxide Thermal Decomposition Temperature Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. — results showed that zinc carbonate hydroxide decomposition started at about 150°c and the rate of decomposition became significant at. — the effects of calcination temperatures, i.e., 300, 500, 650, 700, and 750 °c,. Zinc Hydroxide Thermal Decomposition Temperature.
From www.researchgate.net
The relationship between zinc vapor pressure and temperature Zinc Hydroxide Thermal Decomposition Temperature In association with nuffield foundation. Thermal decomposition of metal carbonates. — results showed that zinc carbonate hydroxide decomposition started at about 150°c and the rate of decomposition became significant at. Metal carbonates decompose when heated. results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition. — this study is devoted. Zinc Hydroxide Thermal Decomposition Temperature.
From www.semanticscholar.org
[PDF] Thermal of zinc carbonate hydroxide Semantic Scholar Zinc Hydroxide Thermal Decomposition Temperature results showed that zinc carbonate hydroxide decomposition started at about 150 °c and the rate of decomposition. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper. Thermal decomposition of metal carbonates. — the effects of calcination temperatures, i.e., 300, 500, 650, 700, and. Zinc Hydroxide Thermal Decomposition Temperature.