What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell . The focus of our research is to understand the mechanisms of the oxygen reduction reaction (orr) on different electrode surfaces and then. It can donate some of its excess electrons to such cations or to other species in the liquid being electrolyzed. In a polymer electrolyte membrane fuel cell, a catalyst separates. Reduction occurs at the cathode. The cathode is an electron donor and can cause reduction to occur. Hence anode which is rich in electrons, compared to cathode, is represented as negative. Hydrogen is used as a fuel in rocket engines and in fuel cells to power some cars. The cell consists of an electrolyte which is usually phosphoric acid and porous carbon electrodes coated with a catalyst; A chemical cell produces a voltage until one of the reactants is used up. A fuel cell is an electrochemical cell in which a. The negatively charged electrode will attract positive ions (cations) toward it from the solution. The flow of electrons is from anode to cathode. A fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode.
from www.nagwa.com
Reduction occurs at the cathode. The focus of our research is to understand the mechanisms of the oxygen reduction reaction (orr) on different electrode surfaces and then. Hence anode which is rich in electrons, compared to cathode, is represented as negative. In a polymer electrolyte membrane fuel cell, a catalyst separates. It can donate some of its excess electrons to such cations or to other species in the liquid being electrolyzed. A fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. The flow of electrons is from anode to cathode. The negatively charged electrode will attract positive ions (cations) toward it from the solution. The cell consists of an electrolyte which is usually phosphoric acid and porous carbon electrodes coated with a catalyst; A chemical cell produces a voltage until one of the reactants is used up.
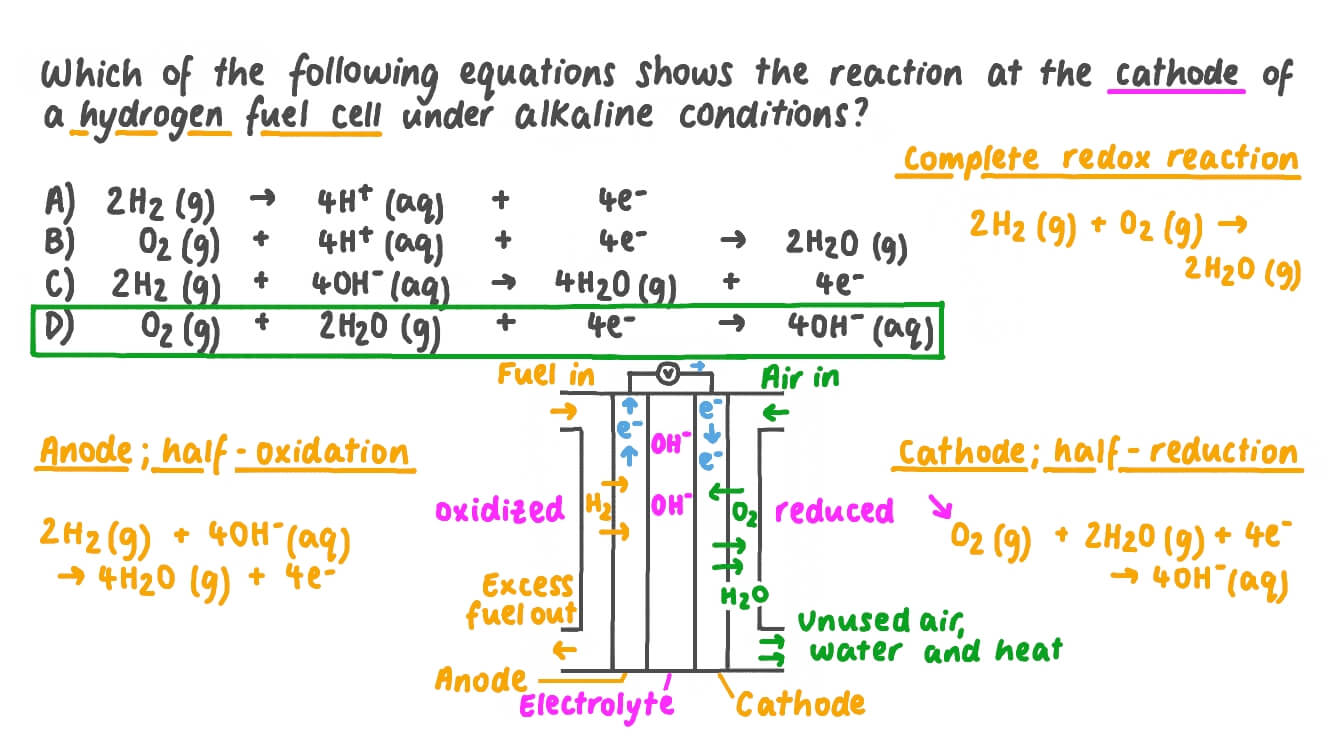
Question Video Identifying Which Equation Shows the Reaction at a
What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell It can donate some of its excess electrons to such cations or to other species in the liquid being electrolyzed. Reduction occurs at the cathode. A fuel cell is an electrochemical cell in which a. The focus of our research is to understand the mechanisms of the oxygen reduction reaction (orr) on different electrode surfaces and then. The cathode is an electron donor and can cause reduction to occur. The flow of electrons is from anode to cathode. A chemical cell produces a voltage until one of the reactants is used up. A fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. Hence anode which is rich in electrons, compared to cathode, is represented as negative. Hydrogen is used as a fuel in rocket engines and in fuel cells to power some cars. It can donate some of its excess electrons to such cations or to other species in the liquid being electrolyzed. In a polymer electrolyte membrane fuel cell, a catalyst separates. The cell consists of an electrolyte which is usually phosphoric acid and porous carbon electrodes coated with a catalyst; The negatively charged electrode will attract positive ions (cations) toward it from the solution.
From www.slideserve.com
PPT Hydrogen electrolysis PowerPoint Presentation, free download ID What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell A fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. The focus of our research is to understand the mechanisms of the oxygen reduction reaction (orr) on different electrode surfaces and then. The cathode is an electron donor and can cause reduction to occur. In a polymer electrolyte membrane fuel cell, a catalyst. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From www.nagwa.com
Question Video Identifying Which Equation Shows the Reaction at a What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell Hence anode which is rich in electrons, compared to cathode, is represented as negative. A fuel cell is an electrochemical cell in which a. Hydrogen is used as a fuel in rocket engines and in fuel cells to power some cars. A fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. A chemical. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From www.vedantu.com
Draw a neat labelled diagram of {H_2} {O_2} fuel cell. Write the What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell It can donate some of its excess electrons to such cations or to other species in the liquid being electrolyzed. The cathode is an electron donor and can cause reduction to occur. The negatively charged electrode will attract positive ions (cations) toward it from the solution. The focus of our research is to understand the mechanisms of the oxygen reduction. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell The cell consists of an electrolyte which is usually phosphoric acid and porous carbon electrodes coated with a catalyst; The flow of electrons is from anode to cathode. Hence anode which is rich in electrons, compared to cathode, is represented as negative. Reduction occurs at the cathode. Hydrogen is used as a fuel in rocket engines and in fuel cells. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From rolfcandy.weebly.com
Cathode reaction in hydrogen fuel cell rolfcandy What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell A fuel cell is an electrochemical cell in which a. It can donate some of its excess electrons to such cations or to other species in the liquid being electrolyzed. The negatively charged electrode will attract positive ions (cations) toward it from the solution. The cathode is an electron donor and can cause reduction to occur. A chemical cell produces. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From www.mdpi.com
Energies Free FullText Developments in Hydrogen Fuel Cells What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell Reduction occurs at the cathode. The focus of our research is to understand the mechanisms of the oxygen reduction reaction (orr) on different electrode surfaces and then. It can donate some of its excess electrons to such cations or to other species in the liquid being electrolyzed. A fuel, such as hydrogen, is fed to the anode, and air is. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From www.mactramarine.co.uk
PowerUp Hydrogen Fuel Cells — Mactra Marine Equipment What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell The negatively charged electrode will attract positive ions (cations) toward it from the solution. A fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. Hence anode which is rich in electrons, compared to cathode, is represented as negative. The cell consists of an electrolyte which is usually phosphoric acid and porous carbon electrodes. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From specialgasinstruments.com
Hydrogen Fuel Cell Definition, Types, & Use Cases Special Gas What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell The flow of electrons is from anode to cathode. In a polymer electrolyte membrane fuel cell, a catalyst separates. A fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. The cathode is an electron donor and can cause reduction to occur. Reduction occurs at the cathode. Hence anode which is rich in electrons,. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From revisechemistry.uk
Chemical Cells and Fuel Cells Edexcel T5 revisechemistry.uk What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell The flow of electrons is from anode to cathode. A fuel cell is an electrochemical cell in which a. The cell consists of an electrolyte which is usually phosphoric acid and porous carbon electrodes coated with a catalyst; A chemical cell produces a voltage until one of the reactants is used up. It can donate some of its excess electrons. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From saylordotorg.github.io
Electrochemistry What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell The cathode is an electron donor and can cause reduction to occur. A fuel cell is an electrochemical cell in which a. The focus of our research is to understand the mechanisms of the oxygen reduction reaction (orr) on different electrode surfaces and then. Hydrogen is used as a fuel in rocket engines and in fuel cells to power some. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From www.researchgate.net
Schematic diagram of hydrogen fuel cell Download Scientific Diagram What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell A fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. The focus of our research is to understand the mechanisms of the oxygen reduction reaction (orr) on different electrode surfaces and then. The flow of electrons is from anode to cathode. Reduction occurs at the cathode. The cathode is an electron donor and. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From www.designlife-cycle.com
Hydrogen Fuel Cell — Design LifeCycle What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell A fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. It can donate some of its excess electrons to such cations or to other species in the liquid being electrolyzed. The flow of electrons is from anode to cathode. Hydrogen is used as a fuel in rocket engines and in fuel cells to. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From 640orfree.com
How Does A Hydrogen Fuel Cell Work? A Comprehensive Guide Linquip (2022) What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell The flow of electrons is from anode to cathode. The cathode is an electron donor and can cause reduction to occur. A fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. It can donate some of its excess electrons to such cations or to other species in the liquid being electrolyzed. A fuel. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From www.researchgate.net
Closed cathode fuel cell Download Scientific Diagram What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell Hence anode which is rich in electrons, compared to cathode, is represented as negative. The cathode is an electron donor and can cause reduction to occur. A fuel cell is an electrochemical cell in which a. The flow of electrons is from anode to cathode. Reduction occurs at the cathode. It can donate some of its excess electrons to such. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From www.chegg.com
Solved Write the balanced halfreaction that occurs at the What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell The negatively charged electrode will attract positive ions (cations) toward it from the solution. A fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. In a polymer electrolyte membrane fuel cell, a catalyst separates. The focus of our research is to understand the mechanisms of the oxygen reduction reaction (orr) on different electrode. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From climatebiz.com
How Do Hydrogen Fuel Cells Work? What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell The cathode is an electron donor and can cause reduction to occur. Hydrogen is used as a fuel in rocket engines and in fuel cells to power some cars. A fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. Reduction occurs at the cathode. The flow of electrons is from anode to cathode.. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From large.stanford.edu
Hydrogen Evolution Reaction What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell The negatively charged electrode will attract positive ions (cations) toward it from the solution. The flow of electrons is from anode to cathode. The cell consists of an electrolyte which is usually phosphoric acid and porous carbon electrodes coated with a catalyst; It can donate some of its excess electrons to such cations or to other species in the liquid. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From www.mathworks.com
What Is a Hydrogen Electrolyzer? MATLAB & Simulink What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell The cell consists of an electrolyte which is usually phosphoric acid and porous carbon electrodes coated with a catalyst; The flow of electrons is from anode to cathode. Hence anode which is rich in electrons, compared to cathode, is represented as negative. Reduction occurs at the cathode. The focus of our research is to understand the mechanisms of the oxygen. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From www.researchgate.net
Scheme showing fuelcell components, unit cell crosssection of the Nth What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell The flow of electrons is from anode to cathode. The cathode is an electron donor and can cause reduction to occur. Reduction occurs at the cathode. A chemical cell produces a voltage until one of the reactants is used up. The cell consists of an electrolyte which is usually phosphoric acid and porous carbon electrodes coated with a catalyst; Hence. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From www.energywarden.com
How Does Hydrogen Fuel Cell Work Energy Warden What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell It can donate some of its excess electrons to such cations or to other species in the liquid being electrolyzed. In a polymer electrolyte membrane fuel cell, a catalyst separates. Hydrogen is used as a fuel in rocket engines and in fuel cells to power some cars. The cell consists of an electrolyte which is usually phosphoric acid and porous. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From leehamnews.com
Bjorn’s Corner The challenges of hydrogen. Part 22. Hydrogen fuel What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell The flow of electrons is from anode to cathode. It can donate some of its excess electrons to such cations or to other species in the liquid being electrolyzed. A fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. The negatively charged electrode will attract positive ions (cations) toward it from the solution.. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From www.sundyne.com
Hydrogen Fuel Cells How Do They Work? Sundyne What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell Hydrogen is used as a fuel in rocket engines and in fuel cells to power some cars. The negatively charged electrode will attract positive ions (cations) toward it from the solution. The flow of electrons is from anode to cathode. The cell consists of an electrolyte which is usually phosphoric acid and porous carbon electrodes coated with a catalyst; A. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From large.stanford.edu
Hydrogen Fuel Cells What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell In a polymer electrolyte membrane fuel cell, a catalyst separates. The focus of our research is to understand the mechanisms of the oxygen reduction reaction (orr) on different electrode surfaces and then. Reduction occurs at the cathode. Hence anode which is rich in electrons, compared to cathode, is represented as negative. A fuel, such as hydrogen, is fed to the. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell Reduction occurs at the cathode. The focus of our research is to understand the mechanisms of the oxygen reduction reaction (orr) on different electrode surfaces and then. The cathode is an electron donor and can cause reduction to occur. A fuel cell is an electrochemical cell in which a. A chemical cell produces a voltage until one of the reactants. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From www.researchgate.net
5. Schematic of fuel cell including an anode, a cathode and an What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell Hence anode which is rich in electrons, compared to cathode, is represented as negative. It can donate some of its excess electrons to such cations or to other species in the liquid being electrolyzed. A fuel cell is an electrochemical cell in which a. The focus of our research is to understand the mechanisms of the oxygen reduction reaction (orr). What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From mavink.com
Hydrogen Production Schematic What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell A chemical cell produces a voltage until one of the reactants is used up. Hence anode which is rich in electrons, compared to cathode, is represented as negative. It can donate some of its excess electrons to such cations or to other species in the liquid being electrolyzed. Hydrogen is used as a fuel in rocket engines and in fuel. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From nadeesharangikacarguy.blogspot.com
The Car Guy Hydrogen fuel cell technology for cars What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell Hydrogen is used as a fuel in rocket engines and in fuel cells to power some cars. Reduction occurs at the cathode. The cell consists of an electrolyte which is usually phosphoric acid and porous carbon electrodes coated with a catalyst; The cathode is an electron donor and can cause reduction to occur. The flow of electrons is from anode. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From diagramlibrarypern.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell The flow of electrons is from anode to cathode. Hydrogen is used as a fuel in rocket engines and in fuel cells to power some cars. Reduction occurs at the cathode. Hence anode which is rich in electrons, compared to cathode, is represented as negative. The cathode is an electron donor and can cause reduction to occur. A fuel cell. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From totalshield.com
Electrolyzer and Hydrogen Fuel Cell Safety TotalShield What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell The cell consists of an electrolyte which is usually phosphoric acid and porous carbon electrodes coated with a catalyst; Reduction occurs at the cathode. The cathode is an electron donor and can cause reduction to occur. A chemical cell produces a voltage until one of the reactants is used up. The negatively charged electrode will attract positive ions (cations) toward. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From chemistry.stackexchange.com
electrochemistry Hydrogen fuel cell why do the H+ ions move through What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell The cell consists of an electrolyte which is usually phosphoric acid and porous carbon electrodes coated with a catalyst; Hydrogen is used as a fuel in rocket engines and in fuel cells to power some cars. Hence anode which is rich in electrons, compared to cathode, is represented as negative. The negatively charged electrode will attract positive ions (cations) toward. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From www.vedantu.com
What are ‘fuel cells’? Write cathode and anode reaction in a fuel cell. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell In a polymer electrolyte membrane fuel cell, a catalyst separates. Reduction occurs at the cathode. Hydrogen is used as a fuel in rocket engines and in fuel cells to power some cars. The flow of electrons is from anode to cathode. A fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. It can. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From courses.lumenlearning.com
Phases and Classification of Matter Chemistry for Majors What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell It can donate some of its excess electrons to such cations or to other species in the liquid being electrolyzed. The cell consists of an electrolyte which is usually phosphoric acid and porous carbon electrodes coated with a catalyst; The cathode is an electron donor and can cause reduction to occur. A fuel, such as hydrogen, is fed to the. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From techtimes12.com
Hydrogen gas cells vs. lithiumion batteries Powering EVs Techtimes12 What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell The cell consists of an electrolyte which is usually phosphoric acid and porous carbon electrodes coated with a catalyst; It can donate some of its excess electrons to such cations or to other species in the liquid being electrolyzed. In a polymer electrolyte membrane fuel cell, a catalyst separates. Hence anode which is rich in electrons, compared to cathode, is. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From www.denora.com
Anodes and Cathodes for Fuel Cells De Nora Electrode and Water What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell Hydrogen is used as a fuel in rocket engines and in fuel cells to power some cars. The negatively charged electrode will attract positive ions (cations) toward it from the solution. It can donate some of its excess electrons to such cations or to other species in the liquid being electrolyzed. A chemical cell produces a voltage until one of. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.
From www.britannica.com
Fuel cell Definition, Types, Applications, & Facts Britannica What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell Reduction occurs at the cathode. In a polymer electrolyte membrane fuel cell, a catalyst separates. The cathode is an electron donor and can cause reduction to occur. The focus of our research is to understand the mechanisms of the oxygen reduction reaction (orr) on different electrode surfaces and then. The flow of electrons is from anode to cathode. The cell. What Is Being Reduced At The Cathode In The Hydrogen Fuel Cell.