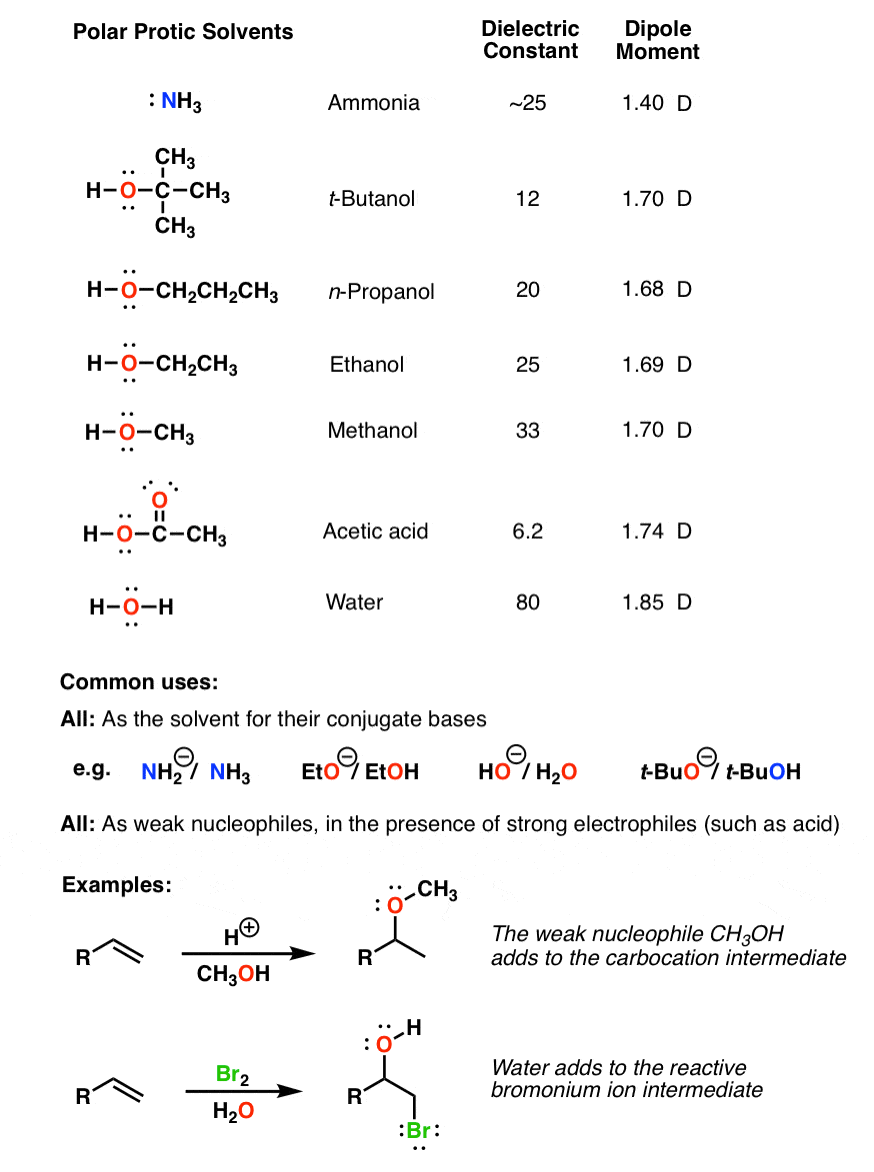
Is Acetone Polar Protic Or Aprotic . 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Take acetone (c 3 h 6 o) for example. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Both are polar but acn is a polar aprotic and metanol a polar protic. Although their dielectric constant are very similar (maybe that is why some researchers suggest the. The main difference between protic and aprotic solvents is their ability to donate hydrogen ions (protons). The choice of solvent can. Acetone is polar because there is a partial negative charge on the oxygen and a partial positive charge on the rest of the molecule. Polar aprotic solvents contain no hydrogen atoms connected directly to an electronegative atom and they are not capable of hydrogen bonding. Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen ions.
from www.masterorganicchemistry.com
The choice of solvent can. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Take acetone (c 3 h 6 o) for example. The main difference between protic and aprotic solvents is their ability to donate hydrogen ions (protons). Although their dielectric constant are very similar (maybe that is why some researchers suggest the. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Acetone is polar because there is a partial negative charge on the oxygen and a partial positive charge on the rest of the molecule. Polar aprotic solvents contain no hydrogen atoms connected directly to an electronegative atom and they are not capable of hydrogen bonding. Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen ions. Both are polar but acn is a polar aprotic and metanol a polar protic.
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents
Is Acetone Polar Protic Or Aprotic Both are polar but acn is a polar aprotic and metanol a polar protic. Both are polar but acn is a polar aprotic and metanol a polar protic. Acetone is polar because there is a partial negative charge on the oxygen and a partial positive charge on the rest of the molecule. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. The choice of solvent can. Although their dielectric constant are very similar (maybe that is why some researchers suggest the. Polar aprotic solvents contain no hydrogen atoms connected directly to an electronegative atom and they are not capable of hydrogen bonding. Take acetone (c 3 h 6 o) for example. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen ions. The main difference between protic and aprotic solvents is their ability to donate hydrogen ions (protons).
From www.pearson.com
The difference between protic vs. aprotic solvents. Pearson+ Channels Is Acetone Polar Protic Or Aprotic 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Take acetone (c 3 h 6 o) for example. Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen ions. Acetone is polar because there is a partial negative charge on the oxygen and. Is Acetone Polar Protic Or Aprotic.
From www.coursehero.com
[Solved] 8. (5 pts. ) We understand phenomena such as solubility in Is Acetone Polar Protic Or Aprotic Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen ions. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Take acetone (c 3 h 6 o) for example. Polar aprotic solvents contain no hydrogen atoms connected directly to an electronegative atom and. Is Acetone Polar Protic Or Aprotic.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID1699463 Is Acetone Polar Protic Or Aprotic 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Take acetone (c 3 h 6 o) for example. Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen ions. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact. Is Acetone Polar Protic Or Aprotic.
From carlynewsturner.blogspot.com
Acetone Polar or Nonpolar Solvent Is Acetone Polar Protic Or Aprotic Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen ions. Acetone is polar because there is a partial negative charge on the oxygen and a partial positive charge on the rest of the molecule. Take acetone (c 3 h 6 o) for example. Polar aprotic solvents contain no hydrogen atoms connected directly to an electronegative atom. Is Acetone Polar Protic Or Aprotic.
From maryi-pubic.blogspot.com
Polar Aprotic Solvents Meaning / Nucleophilic Substitution And Is Acetone Polar Protic Or Aprotic Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen ions. Take acetone (c 3 h 6 o) for example. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Although their dielectric constant are very similar (maybe that is why some researchers suggest. Is Acetone Polar Protic Or Aprotic.
From ar.inspiredpencil.com
Acetone Lewis Structure With Polarity Is Acetone Polar Protic Or Aprotic Acetone is polar because there is a partial negative charge on the oxygen and a partial positive charge on the rest of the molecule. Polar aprotic solvents contain no hydrogen atoms connected directly to an electronegative atom and they are not capable of hydrogen bonding. The choice of solvent can. The main difference between protic and aprotic solvents is their. Is Acetone Polar Protic Or Aprotic.
From www.youtube.com
What is the difference between polar protic and aprotic solvents? YouTube Is Acetone Polar Protic Or Aprotic Polar aprotic solvents contain no hydrogen atoms connected directly to an electronegative atom and they are not capable of hydrogen bonding. Acetone is polar because there is a partial negative charge on the oxygen and a partial positive charge on the rest of the molecule. Both are polar but acn is a polar aprotic and metanol a polar protic. Protic. Is Acetone Polar Protic Or Aprotic.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Master Is Acetone Polar Protic Or Aprotic The main difference between protic and aprotic solvents is their ability to donate hydrogen ions (protons). 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Acetone is polar because there is a partial negative charge on the oxygen and a partial positive charge on the rest. Is Acetone Polar Protic Or Aprotic.
From psiberg.com
Protic vs Aprotic Solvents (with Examples) PSIBERG Is Acetone Polar Protic Or Aprotic Both are polar but acn is a polar aprotic and metanol a polar protic. The choice of solvent can. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Although their dielectric constant are very similar (maybe that is why some researchers suggest the. The main difference. Is Acetone Polar Protic Or Aprotic.
From www.transtutors.com
(Solved) . Label Each Solvent As Nonpolar, Polar Protic Or Polar Is Acetone Polar Protic Or Aprotic Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen ions. The choice of solvent can. Although their dielectric constant are very similar (maybe that is why some researchers suggest the. Take acetone (c 3 h 6 o) for example. The main difference between protic and aprotic solvents is their ability to donate hydrogen ions (protons). 23. Is Acetone Polar Protic Or Aprotic.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Is Acetone Polar Protic Or Aprotic Take acetone (c 3 h 6 o) for example. The main difference between protic and aprotic solvents is their ability to donate hydrogen ions (protons). 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate. Is Acetone Polar Protic Or Aprotic.
From www.numerade.com
SOLVEDIdentify if the following polar solvents are protic or aprotic Is Acetone Polar Protic Or Aprotic Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen ions. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. The main difference between protic and aprotic solvents is their ability to donate hydrogen ions (protons). The choice of solvent can. Both are. Is Acetone Polar Protic Or Aprotic.
From www.coursehero.com
[Solved] What is the difference between polar protic and polar aprotic Is Acetone Polar Protic Or Aprotic Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen ions. Both are polar but acn is a polar aprotic and metanol a polar protic. Acetone is polar because there is a partial negative charge on the oxygen and a partial positive charge on the rest of the molecule. The main difference between protic and aprotic solvents. Is Acetone Polar Protic Or Aprotic.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID375520 Is Acetone Polar Protic Or Aprotic 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Both are polar but acn is a polar aprotic and metanol a polar protic. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less.. Is Acetone Polar Protic Or Aprotic.
From chemistrytalk.org
Polar Protic and Aprotic Solvents ChemTalk Is Acetone Polar Protic Or Aprotic 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Take acetone (c 3 h 6 o) for example. The choice of solvent can. Both are polar but acn is a polar aprotic and metanol a polar protic. 23 rows however, acetone is still considered a polar. Is Acetone Polar Protic Or Aprotic.
From answerlibaccuses.z21.web.core.windows.net
Acetone Is Polar Protic Or Aprotic Is Acetone Polar Protic Or Aprotic The main difference between protic and aprotic solvents is their ability to donate hydrogen ions (protons). Polar aprotic solvents contain no hydrogen atoms connected directly to an electronegative atom and they are not capable of hydrogen bonding. Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen ions. Both are polar but acn is a polar aprotic. Is Acetone Polar Protic Or Aprotic.
From chemistrytalk.org
Polar Protic and Aprotic Solvents ChemTalk Is Acetone Polar Protic Or Aprotic Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen ions. Acetone is polar because there is a partial negative charge on the oxygen and a partial positive charge on the rest of the molecule. Polar aprotic solvents contain no hydrogen atoms connected directly to an electronegative atom and they are not capable of hydrogen bonding. Take. Is Acetone Polar Protic Or Aprotic.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID6758085 Is Acetone Polar Protic Or Aprotic Take acetone (c 3 h 6 o) for example. Although their dielectric constant are very similar (maybe that is why some researchers suggest the. Polar aprotic solvents contain no hydrogen atoms connected directly to an electronegative atom and they are not capable of hydrogen bonding. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that. Is Acetone Polar Protic Or Aprotic.
From copyprogramming.com
Understanding the Distinctions between Protic and Aprotic Solvents Is Acetone Polar Protic Or Aprotic Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen ions. The main difference between protic and aprotic solvents is their ability to donate hydrogen ions (protons). Although their dielectric constant are very similar (maybe that is why some researchers suggest the. Take acetone (c 3 h 6 o) for example. Polar aprotic solvents contain no hydrogen. Is Acetone Polar Protic Or Aprotic.
From www.youtube.com
maxresdefault.jpg Is Acetone Polar Protic Or Aprotic 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. The choice of solvent can. Although their dielectric constant are very similar (maybe that is why some researchers suggest the. The main difference between protic and aprotic solvents is their ability to donate hydrogen ions (protons). 23. Is Acetone Polar Protic Or Aprotic.
From answerlibaccuses.z21.web.core.windows.net
Acetone Is Polar Protic Or Aprotic Is Acetone Polar Protic Or Aprotic Polar aprotic solvents contain no hydrogen atoms connected directly to an electronegative atom and they are not capable of hydrogen bonding. The main difference between protic and aprotic solvents is their ability to donate hydrogen ions (protons). Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen ions. Acetone is polar because there is a partial negative. Is Acetone Polar Protic Or Aprotic.
From edu.svet.gob.gt
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Is Acetone Polar Protic Or Aprotic The choice of solvent can. Acetone is polar because there is a partial negative charge on the oxygen and a partial positive charge on the rest of the molecule. Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen ions. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is. Is Acetone Polar Protic Or Aprotic.
From organicchemistoncall.com
SN2 versus SN1 Organic Chemist On Call Is Acetone Polar Protic Or Aprotic Both are polar but acn is a polar aprotic and metanol a polar protic. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Take acetone (c 3 h 6 o) for example. Although their dielectric constant are very similar (maybe that is why some researchers suggest. Is Acetone Polar Protic Or Aprotic.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Is Acetone Polar Protic Or Aprotic Take acetone (c 3 h 6 o) for example. Although their dielectric constant are very similar (maybe that is why some researchers suggest the. Polar aprotic solvents contain no hydrogen atoms connected directly to an electronegative atom and they are not capable of hydrogen bonding. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that. Is Acetone Polar Protic Or Aprotic.
From www.youtube.com
Polar protic vs. polar aprotic solvents YouTube Is Acetone Polar Protic Or Aprotic The main difference between protic and aprotic solvents is their ability to donate hydrogen ions (protons). Both are polar but acn is a polar aprotic and metanol a polar protic. Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen ions. Polar aprotic solvents contain no hydrogen atoms connected directly to an electronegative atom and they are. Is Acetone Polar Protic Or Aprotic.
From www.chemistrysteps.com
The Role of Solvent in SN1, SN2, E1 and E2 Reactions Chemistry Steps Is Acetone Polar Protic Or Aprotic Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen ions. Although their dielectric constant are very similar (maybe that is why some researchers suggest the. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. The main difference between protic and aprotic solvents. Is Acetone Polar Protic Or Aprotic.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Master Is Acetone Polar Protic Or Aprotic Both are polar but acn is a polar aprotic and metanol a polar protic. Although their dielectric constant are very similar (maybe that is why some researchers suggest the. Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen ions. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is. Is Acetone Polar Protic Or Aprotic.
From www.slideserve.com
PPT Substitution and Elimination PowerPoint Presentation, free Is Acetone Polar Protic Or Aprotic 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Take acetone (c 3 h 6 o) for example. Acetone is polar because there. Is Acetone Polar Protic Or Aprotic.
From www.pinterest.com
Polar aprotic solvents favor SN2 while polar protic solvents favor the Is Acetone Polar Protic Or Aprotic Although their dielectric constant are very similar (maybe that is why some researchers suggest the. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Take acetone (c 3 h 6 o) for example. Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen. Is Acetone Polar Protic Or Aprotic.
From www.pinterest.co.kr
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Organic Is Acetone Polar Protic Or Aprotic Acetone is polar because there is a partial negative charge on the oxygen and a partial positive charge on the rest of the molecule. Both are polar but acn is a polar aprotic and metanol a polar protic. The main difference between protic and aprotic solvents is their ability to donate hydrogen ions (protons). Although their dielectric constant are very. Is Acetone Polar Protic Or Aprotic.
From socratic.org
Polar Protic, Polar Aprotic and NonPolar Solvents Organic Chemistry Is Acetone Polar Protic Or Aprotic 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Both are polar but acn is a polar aprotic and metanol a polar protic. The main difference between protic and aprotic solvents is their ability to donate hydrogen ions (protons). The choice of solvent can. Protic solvents. Is Acetone Polar Protic Or Aprotic.
From www.pearson.com
The difference between protic vs. aprotic solvents. Pearson+ Channels Is Acetone Polar Protic Or Aprotic Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen ions. Take acetone (c 3 h 6 o) for example. The choice of solvent can. Both are polar but acn is a polar aprotic and metanol a polar protic. Polar aprotic solvents contain no hydrogen atoms connected directly to an electronegative atom and they are not capable. Is Acetone Polar Protic Or Aprotic.
From www.difference101.com
Protic vs Aprotic Solvents 11 Key Differences To Know Difference 101 Is Acetone Polar Protic Or Aprotic Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen ions. Acetone is polar because there is a partial negative charge on the oxygen and a partial positive charge on the rest of the molecule. The main difference between protic and aprotic solvents is their ability to donate hydrogen ions (protons). 23 rows however, acetone is still. Is Acetone Polar Protic Or Aprotic.
From chem-net.blogspot.com
Solvent Effects and SN2 and SN1 reactions Nucleophilic Substitution Is Acetone Polar Protic Or Aprotic Take acetone (c 3 h 6 o) for example. Both are polar but acn is a polar aprotic and metanol a polar protic. Although their dielectric constant are very similar (maybe that is why some researchers suggest the. Protic solvents can donate hydrogen ions (protons), but aprotic solvents cannot donate hydrogen ions. Polar aprotic solvents contain no hydrogen atoms connected. Is Acetone Polar Protic Or Aprotic.
From www.slideserve.com
PPT 11. Reactions of Alkyl Halides Nucleophilic Substitutions and Is Acetone Polar Protic Or Aprotic The main difference between protic and aprotic solvents is their ability to donate hydrogen ions (protons). Take acetone (c 3 h 6 o) for example. Both are polar but acn is a polar aprotic and metanol a polar protic. Acetone is polar because there is a partial negative charge on the oxygen and a partial positive charge on the rest. Is Acetone Polar Protic Or Aprotic.