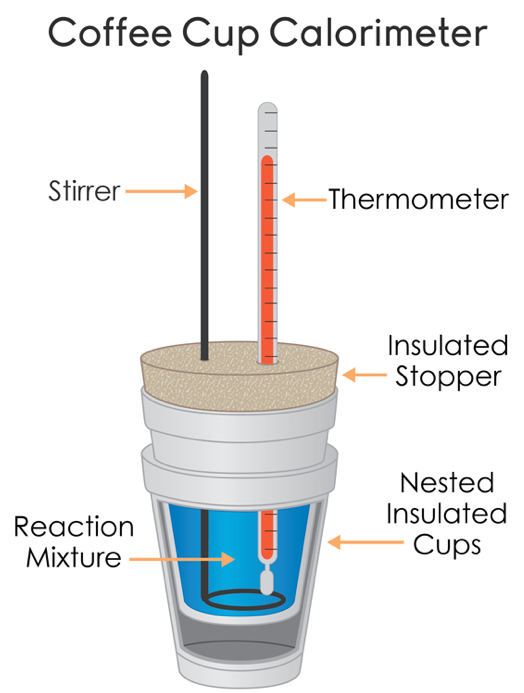
What Is A Calorimeter And Its Use . For example, when an exothermic. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is applied extensively in the fields of thermochemistry in calculating. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to determine the amount of heat. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. It uses devices called calorimeters, which measure the change in. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter is what is used to measure the thermal changes of a body.
from physique.ensc-rennes.fr
A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. For example, when an exothermic. It uses devices called calorimeters, which measure the change in. Calorimetry is applied extensively in the fields of thermochemistry in calculating. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is what is used to measure the thermal changes of a body. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Chemists use calorimetry to determine the amount of heat.
TP calorimetry
What Is A Calorimeter And Its Use Chemists use calorimetry to determine the amount of heat. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Chemists use calorimetry to determine the amount of heat. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. For example, when an exothermic. Calorimetry is applied extensively in the fields of thermochemistry in calculating. A calorimeter is what is used to measure the thermal changes of a body. It uses devices called calorimeters, which measure the change in.
From www.youtube.com
050 Calorimetry YouTube What Is A Calorimeter And Its Use Chemists use calorimetry to determine the amount of heat. For example, when an exothermic. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. It uses devices called calorimeters, which measure the change in. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features. What Is A Calorimeter And Its Use.
From www.excedr.com
What Is a Calorimeter & How Is It Used in a Lab? What Is A Calorimeter And Its Use A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is applied extensively in the fields of thermochemistry in calculating. Chemists use calorimetry to determine the amount of heat. Calorimetry is a branch of science. What Is A Calorimeter And Its Use.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation What Is A Calorimeter And Its Use To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Chemists use calorimetry to determine the amount of heat. It uses devices called calorimeters, which measure the change in. For example, when an exothermic. A calorimeter is what is used to measure the thermal changes of a body. Calorimetry is the set of techniques used. What Is A Calorimeter And Its Use.
From www.walmart.com
Electric Calorimeter What Is A Calorimeter And Its Use Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It uses devices called calorimeters, which measure the change in. A calorimeter is what is used to measure the thermal changes of. What Is A Calorimeter And Its Use.
From faqguide.co
What does a calorimeter do? Explained by FAQGuide What Is A Calorimeter And Its Use Chemists use calorimetry to determine the amount of heat. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is applied extensively in the fields of thermochemistry in calculating. It uses devices called calorimeters, which measure the change in. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a. What Is A Calorimeter And Its Use.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry What Is A Calorimeter And Its Use To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is applied extensively in the fields of thermochemistry in calculating. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is what is used to measure the thermal changes of a body. Chemists. What Is A Calorimeter And Its Use.
From engineeringlearn.com
Bomb Calorimeter Definition, Construction, Diagram, Working & Uses What Is A Calorimeter And Its Use Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is applied extensively in the fields. What Is A Calorimeter And Its Use.
From physique.ensc-rennes.fr
TP calorimetry What Is A Calorimeter And Its Use It uses devices called calorimeters, which measure the change in. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is. What Is A Calorimeter And Its Use.
From www.pinterest.com.au
Pin on Science Friday What Is A Calorimeter And Its Use Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter is what is used to measure the thermal changes of a body. Chemists use calorimetry to determine the amount of heat. For example, when an exothermic. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. What Is A Calorimeter And Its Use.
From www.techraj6.com
what is Calorimeter What Is A Calorimeter And Its Use Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Chemists use calorimetry to determine the amount of heat. For example, when. What Is A Calorimeter And Its Use.
From study.com
Calorimetry Definition, Equation & Types Lesson What Is A Calorimeter And Its Use Calorimetry is applied extensively in the fields of thermochemistry in calculating. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Chemists use calorimetry to determine the amount of heat. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is a branch of science. What Is A Calorimeter And Its Use.
From drivenheisenberg.blogspot.com
Which Diagram Is A Bomb Calorimeter Drivenheisenberg What Is A Calorimeter And Its Use To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Chemists use calorimetry to determine the amount of heat. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is. What Is A Calorimeter And Its Use.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle What Is A Calorimeter And Its Use For example, when an exothermic. Chemists use calorimetry to determine the amount of heat. It uses devices called calorimeters, which measure the change in. Calorimetry is applied extensively in the fields of thermochemistry in calculating. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a field of thermochemistry that measures the amount. What Is A Calorimeter And Its Use.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas What Is A Calorimeter And Its Use It uses devices called calorimeters, which measure the change in. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. For example, when an exothermic. Chemists use calorimetry to determine the amount of heat. Calorimetry is applied. What Is A Calorimeter And Its Use.
From www.techraj6.com
what is Calorimeter What Is A Calorimeter And Its Use To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter is a device used to. What Is A Calorimeter And Its Use.
From www.britannica.com
Calorimeter instrument Britannica What Is A Calorimeter And Its Use Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is applied extensively in the fields of thermochemistry in calculating. Calorimetry is the set of techniques. What Is A Calorimeter And Its Use.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson What Is A Calorimeter And Its Use Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. It uses devices called calorimeters, which measure the change in. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is. What Is A Calorimeter And Its Use.
From www.chegg.com
Solved PRELAB ASSIGNMENT 1. What is a calorimeter? What is What Is A Calorimeter And Its Use Calorimetry is applied extensively in the fields of thermochemistry in calculating. Chemists use calorimetry to determine the amount of heat. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a field of thermochemistry. What Is A Calorimeter And Its Use.
From www.youtube.com
Principle of Calorimetry YouTube What Is A Calorimeter And Its Use For example, when an exothermic. It uses devices called calorimeters, which measure the change in. Calorimetry is applied extensively in the fields of thermochemistry in calculating. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. To. What Is A Calorimeter And Its Use.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry What Is A Calorimeter And Its Use Chemists use calorimetry to determine the amount of heat. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It uses devices called calorimeters, which measure the change in. For example, when an exothermic. Calorimetry is. What Is A Calorimeter And Its Use.
From labsuppliesusa.com
Calorimeter Electric KLM Bio Scientific What Is A Calorimeter And Its Use Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Chemists use calorimetry to determine the amount of heat. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is a. What Is A Calorimeter And Its Use.
From ecampusontario.pressbooks.pub
3.5 Calorimétrie La Chimie Générale pour les GeeGees What Is A Calorimeter And Its Use To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. It uses devices called calorimeters, which measure the change in. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter is what is used to measure the thermal changes of a body. Calorimetry is a branch of science concerned. What Is A Calorimeter And Its Use.
From www.freeastroscience.com
Exploring Calorimetry A Journey into Heat Transfer Measurement What Is A Calorimeter And Its Use A calorimeter is what is used to measure the thermal changes of a body. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. For example, when an exothermic. Chemists use calorimetry to determine the amount of heat. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical. What Is A Calorimeter And Its Use.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 What Is A Calorimeter And Its Use Chemists use calorimetry to determine the amount of heat. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. A calorimeter is what is used to measure the thermal changes of a body. Calorimetry is the. What Is A Calorimeter And Its Use.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube What Is A Calorimeter And Its Use Calorimetry is applied extensively in the fields of thermochemistry in calculating. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal. What Is A Calorimeter And Its Use.
From www.sciencephoto.com
Parts of a Bomb Calorimeter Stock Image C002/8063 Science Photo What Is A Calorimeter And Its Use A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Chemists use calorimetry to determine the amount of heat. It uses devices called calorimeters, which measure the change in. Calorimetry is. What Is A Calorimeter And Its Use.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 What Is A Calorimeter And Its Use Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is applied extensively in the fields of thermochemistry in calculating. A calorimeter is what is used to measure the thermal changes of a body. A calorimeter is a device used to measure the amount of heat involved in a chemical. What Is A Calorimeter And Its Use.
From saylordotorg.github.io
Calorimetry What Is A Calorimeter And Its Use Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is what is used to measure the thermal changes of a body. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is applied extensively in the fields of thermochemistry in calculating. Calorimetry. What Is A Calorimeter And Its Use.
From www.techraj6.com
what is Calorimeter What Is A Calorimeter And Its Use Chemists use calorimetry to determine the amount of heat. A calorimeter is what is used to measure the thermal changes of a body. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimetry is applied extensively in the fields of thermochemistry in calculating. It uses. What Is A Calorimeter And Its Use.
From saylordotorg.github.io
Calorimetry What Is A Calorimeter And Its Use Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. For example, when an exothermic. A calorimeter is what is used to measure the thermal changes of. What Is A Calorimeter And Its Use.
From www.youtube.com
Calorimetry Part II Lab Version) YouTube What Is A Calorimeter And Its Use To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example,. What Is A Calorimeter And Its Use.
From www.laboratory-equipment.com
Calorimeter Features, Styles, Types, and Differences What Is A Calorimeter And Its Use A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is applied extensively in the fields of thermochemistry in calculating. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Chemists use calorimetry to determine the amount of heat. A calorimeter is what is used. What Is A Calorimeter And Its Use.
From www.thoughtco.com
Calorimeter Definition in Chemistry What Is A Calorimeter And Its Use Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is applied extensively in the fields of thermochemistry in calculating. A calorimeter is what is used to measure the thermal. What Is A Calorimeter And Its Use.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec What Is A Calorimeter And Its Use Calorimetry is applied extensively in the fields of thermochemistry in calculating. It uses devices called calorimeters, which measure the change in. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Chemists. What Is A Calorimeter And Its Use.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry What Is A Calorimeter And Its Use Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Chemists use calorimetry to determine the amount of heat. A calorimeter is a device used to measure the amount of heat involved. What Is A Calorimeter And Its Use.