Electrodes Concepts Chemistry . Describe and relate the definitions of electrode and cell potentials. Electrochemistry is the study of chemical processes that cause electrons to move. Interpret electrode potentials in terms of relative oxidant and reductant. Interpret electrode potentials in terms of relative oxidant and reductant strengths. To understand electrochemistry we need to appreciate five important and interrelated concepts: The reaction can be started and stopped by connecting or disconnecting the two electrodes. The book outlines the most important theoretical concepts and provides a fairly detailed derivation of the basic formulas and equations. Electrochemical cells allow measurement and control of a redox reaction. The electrode’s potential determines the analyte’s form at the. An electrochemical cell consists of two electrically conductive electrodes immersed or in contact with an electrolyte (fig. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Describe and relate the definitions of electrode and cell potentials. This movement of electrons is called.
from www.youtube.com
Electrochemical cells allow measurement and control of a redox reaction. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Describe and relate the definitions of electrode and cell potentials. This movement of electrons is called. The book outlines the most important theoretical concepts and provides a fairly detailed derivation of the basic formulas and equations. Electrochemistry is the study of chemical processes that cause electrons to move. An electrochemical cell consists of two electrically conductive electrodes immersed or in contact with an electrolyte (fig. Interpret electrode potentials in terms of relative oxidant and reductant. The electrode’s potential determines the analyte’s form at the. Describe and relate the definitions of electrode and cell potentials.
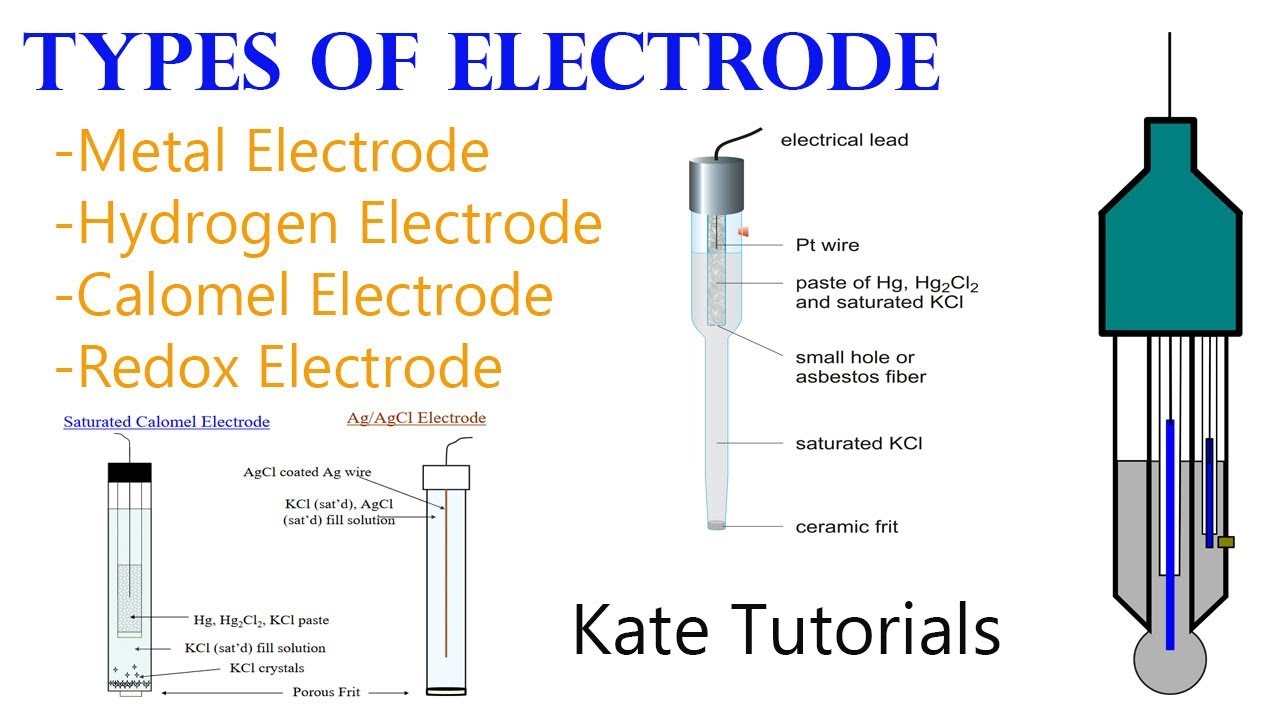
6 Different Types of Electrodes & their Reactions in Electrochemistry
Electrodes Concepts Chemistry Electrochemical cells allow measurement and control of a redox reaction. Interpret electrode potentials in terms of relative oxidant and reductant. The electrode’s potential determines the analyte’s form at the. The book outlines the most important theoretical concepts and provides a fairly detailed derivation of the basic formulas and equations. Describe and relate the definitions of electrode and cell potentials. The reaction can be started and stopped by connecting or disconnecting the two electrodes. This movement of electrons is called. Electrochemical cells allow measurement and control of a redox reaction. Electrochemistry is the study of chemical processes that cause electrons to move. To understand electrochemistry we need to appreciate five important and interrelated concepts: Interpret electrode potentials in terms of relative oxidant and reductant strengths. Describe and relate the definitions of electrode and cell potentials. An electrochemical cell consists of two electrically conductive electrodes immersed or in contact with an electrolyte (fig. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most.
From saylordotorg.github.io
Electrochemistry Electrodes Concepts Chemistry Describe and relate the definitions of electrode and cell potentials. Electrochemical cells allow measurement and control of a redox reaction. This movement of electrons is called. Interpret electrode potentials in terms of relative oxidant and reductant. Electrochemistry is the study of chemical processes that cause electrons to move. The electrode’s potential determines the analyte’s form at the. To understand electrochemistry. Electrodes Concepts Chemistry.
From philschatz.com
Electrolytes · Chemistry Electrodes Concepts Chemistry The book outlines the most important theoretical concepts and provides a fairly detailed derivation of the basic formulas and equations. Electrochemistry is the study of chemical processes that cause electrons to move. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. The electrode’s potential determines the analyte’s form at. Electrodes Concepts Chemistry.
From chem.libretexts.org
Electrolysis I Chemistry LibreTexts Electrodes Concepts Chemistry Electrochemistry is the study of chemical processes that cause electrons to move. Describe and relate the definitions of electrode and cell potentials. Electrochemical cells allow measurement and control of a redox reaction. To understand electrochemistry we need to appreciate five important and interrelated concepts: The reaction can be started and stopped by connecting or disconnecting the two electrodes. An electrochemical. Electrodes Concepts Chemistry.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Electrodes Concepts Chemistry Electrochemical cells allow measurement and control of a redox reaction. To understand electrochemistry we need to appreciate five important and interrelated concepts: Interpret electrode potentials in terms of relative oxidant and reductant strengths. The electrode’s potential determines the analyte’s form at the. This movement of electrons is called. Interpret electrode potentials in terms of relative oxidant and reductant. The book. Electrodes Concepts Chemistry.
From www.youtube.com
General Concepts of Chemistry Method to determine Standard Electrode Electrodes Concepts Chemistry Electrochemical cells allow measurement and control of a redox reaction. Describe and relate the definitions of electrode and cell potentials. An electrochemical cell consists of two electrically conductive electrodes immersed or in contact with an electrolyte (fig. The reaction can be started and stopped by connecting or disconnecting the two electrodes. Describe and relate the definitions of electrode and cell. Electrodes Concepts Chemistry.
From greenenergymaterial.com
What Is Electrode Potential And Their Applications » Green Energy Material Electrodes Concepts Chemistry This movement of electrons is called. The electrode’s potential determines the analyte’s form at the. The book outlines the most important theoretical concepts and provides a fairly detailed derivation of the basic formulas and equations. The reaction can be started and stopped by connecting or disconnecting the two electrodes. Interpret electrode potentials in terms of relative oxidant and reductant. Electrochemistry. Electrodes Concepts Chemistry.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrodes Concepts Chemistry Describe and relate the definitions of electrode and cell potentials. Electrochemistry is the study of chemical processes that cause electrons to move. The electrode’s potential determines the analyte’s form at the. Electrochemical cells allow measurement and control of a redox reaction. The book outlines the most important theoretical concepts and provides a fairly detailed derivation of the basic formulas and. Electrodes Concepts Chemistry.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo Electrodes Concepts Chemistry Interpret electrode potentials in terms of relative oxidant and reductant. The book outlines the most important theoretical concepts and provides a fairly detailed derivation of the basic formulas and equations. The electrode’s potential determines the analyte’s form at the. Describe and relate the definitions of electrode and cell potentials. Describe and relate the definitions of electrode and cell potentials. Electrochemistry. Electrodes Concepts Chemistry.
From www.studypool.com
SOLUTION Electrochemistry Complete Chapter with Electrochemical Electrodes Concepts Chemistry To understand electrochemistry we need to appreciate five important and interrelated concepts: Interpret electrode potentials in terms of relative oxidant and reductant. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The electrode’s potential determines the analyte’s form at the. Electrochemistry is the study of chemical processes that cause electrons to move. Electrochemical cells allow measurement and control. Electrodes Concepts Chemistry.
From www.semanticscholar.org
Figure 1 from Rational design of allsolidstate ionselective Electrodes Concepts Chemistry The book outlines the most important theoretical concepts and provides a fairly detailed derivation of the basic formulas and equations. This movement of electrons is called. Electrochemical cells allow measurement and control of a redox reaction. Describe and relate the definitions of electrode and cell potentials. The electrode’s potential determines the analyte’s form at the. An electrochemical cell consists of. Electrodes Concepts Chemistry.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Electrodes Concepts Chemistry Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. This movement of electrons is called. The electrode’s potential determines the analyte’s form at the. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. An electrochemical cell consists. Electrodes Concepts Chemistry.
From alevelchemistry.co.uk
Electrodes Facts, Summary & Definition Chemistry Revision Electrodes Concepts Chemistry Interpret electrode potentials in terms of relative oxidant and reductant strengths. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. To understand electrochemistry we need to appreciate five important and interrelated concepts: Describe and relate the definitions of electrode and cell potentials. Electrochemical cells allow measurement and control of. Electrodes Concepts Chemistry.
From question.pandai.org
Standard Electrode Potential Electrodes Concepts Chemistry The reaction can be started and stopped by connecting or disconnecting the two electrodes. This movement of electrons is called. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. Electrochemical cells allow measurement and control of a redox. Electrodes Concepts Chemistry.
From www.snexplores.org
Explainer What is an electrode? Electrodes Concepts Chemistry Describe and relate the definitions of electrode and cell potentials. An electrochemical cell consists of two electrically conductive electrodes immersed or in contact with an electrolyte (fig. Interpret electrode potentials in terms of relative oxidant and reductant. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Electrochemical cells allow measurement and control of a redox reaction. The book. Electrodes Concepts Chemistry.
From www.youtube.com
IonSelective Electrode, Principle, Advantages, Disadvantages Electrodes Concepts Chemistry The electrode’s potential determines the analyte’s form at the. This movement of electrons is called. The book outlines the most important theoretical concepts and provides a fairly detailed derivation of the basic formulas and equations. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Electrochemistry is the study of. Electrodes Concepts Chemistry.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Electrodes Concepts Chemistry This movement of electrons is called. The book outlines the most important theoretical concepts and provides a fairly detailed derivation of the basic formulas and equations. The reaction can be started and stopped by connecting or disconnecting the two electrodes. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Describe and relate the definitions of electrode and cell. Electrodes Concepts Chemistry.
From mantavya.com
What Is Electroplating & How does it work 2021 Guide Mantavya Electrodes Concepts Chemistry This movement of electrons is called. The electrode’s potential determines the analyte’s form at the. An electrochemical cell consists of two electrically conductive electrodes immersed or in contact with an electrolyte (fig. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The reaction can be started and stopped by connecting or disconnecting the two electrodes. Describe and relate. Electrodes Concepts Chemistry.
From www.researchgate.net
Schematic illustration of a typical three‐electrode system. Download Electrodes Concepts Chemistry Electrochemistry is the study of chemical processes that cause electrons to move. An electrochemical cell consists of two electrically conductive electrodes immersed or in contact with an electrolyte (fig. Describe and relate the definitions of electrode and cell potentials. The reaction can be started and stopped by connecting or disconnecting the two electrodes. This movement of electrons is called. Interpret. Electrodes Concepts Chemistry.
From saylordotorg.github.io
Electrochemistry Electrodes Concepts Chemistry Describe and relate the definitions of electrode and cell potentials. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The reaction can be started and stopped by connecting or disconnecting the two electrodes. To understand electrochemistry we need. Electrodes Concepts Chemistry.
From www.youtube.com
What are different types of Reversible Electrodes? Electrochemistry Electrodes Concepts Chemistry The book outlines the most important theoretical concepts and provides a fairly detailed derivation of the basic formulas and equations. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Describe and relate the definitions of electrode and cell potentials. The reaction can be started and stopped by connecting or disconnecting the two electrodes. This movement of electrons is. Electrodes Concepts Chemistry.
From slidetodoc.com
ION SELECTIVE ELECTRODES In Brief OUTLINES Introduction Reference Electrodes Concepts Chemistry To understand electrochemistry we need to appreciate five important and interrelated concepts: Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Electrochemistry is the study of chemical processes that cause electrons to move. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of. Electrodes Concepts Chemistry.
From www.youtube.com
General Concepts of Chemistry Standard Electrode Potential YouTube Electrodes Concepts Chemistry Interpret electrode potentials in terms of relative oxidant and reductant. The reaction can be started and stopped by connecting or disconnecting the two electrodes. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. To understand electrochemistry we need to appreciate five important and interrelated concepts: Electrochemical cells allow measurement. Electrodes Concepts Chemistry.
From www.pinterest.com
CATHODE RAY TUBE CROOKE'S TUBE Electrons move from the negative Electrodes Concepts Chemistry Describe and relate the definitions of electrode and cell potentials. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Interpret electrode potentials in terms of relative oxidant and reductant. This movement of electrons is called. Electrochemical cells allow measurement and control of a redox reaction. The electrode’s potential determines. Electrodes Concepts Chemistry.
From www.youtube.com
Cathode and Anode Quick differences and comparisons YouTube Electrodes Concepts Chemistry Electrochemistry is the study of chemical processes that cause electrons to move. The reaction can be started and stopped by connecting or disconnecting the two electrodes. An electrochemical cell consists of two electrically conductive electrodes immersed or in contact with an electrolyte (fig. Describe and relate the definitions of electrode and cell potentials. Electrode reactions are a class of chemical. Electrodes Concepts Chemistry.
From mungfali.com
Electrode Potential Series Electrodes Concepts Chemistry Electrochemical cells allow measurement and control of a redox reaction. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Interpret electrode potentials in terms of relative oxidant and reductant. The reaction can be started and stopped by connecting or disconnecting the two electrodes. The book outlines the most important theoretical concepts and provides a fairly detailed derivation of. Electrodes Concepts Chemistry.
From chem.libretexts.org
1.7 Ion Selective Electrode Analysis Chemistry LibreTexts Electrodes Concepts Chemistry Electrochemistry is the study of chemical processes that cause electrons to move. An electrochemical cell consists of two electrically conductive electrodes immersed or in contact with an electrolyte (fig. The book outlines the most important theoretical concepts and provides a fairly detailed derivation of the basic formulas and equations. Interpret electrode potentials in terms of relative oxidant and reductant. Electrochemical. Electrodes Concepts Chemistry.
From semcouniversity.com
How the three electrode system works Semco University Semco Electrodes Concepts Chemistry An electrochemical cell consists of two electrically conductive electrodes immersed or in contact with an electrolyte (fig. This movement of electrons is called. The electrode’s potential determines the analyte’s form at the. Interpret electrode potentials in terms of relative oxidant and reductant. The book outlines the most important theoretical concepts and provides a fairly detailed derivation of the basic formulas. Electrodes Concepts Chemistry.
From www.researchgate.net
Electrochemical analysis threeelectrode setup. Schematic of the Electrodes Concepts Chemistry Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Describe and relate the definitions of electrode and cell potentials. The reaction can be started and stopped by connecting or disconnecting the two electrodes. Electrochemical cells allow measurement and control of a redox reaction. Interpret electrode potentials in terms of. Electrodes Concepts Chemistry.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrodes Concepts Chemistry Electrochemistry is the study of chemical processes that cause electrons to move. Electrochemical cells allow measurement and control of a redox reaction. Describe and relate the definitions of electrode and cell potentials. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Interpret electrode potentials in terms of relative oxidant. Electrodes Concepts Chemistry.
From chem.libretexts.org
20.2 Standard Electrode Potentials Chemistry LibreTexts Electrodes Concepts Chemistry Electrochemistry is the study of chemical processes that cause electrons to move. An electrochemical cell consists of two electrically conductive electrodes immersed or in contact with an electrolyte (fig. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. This movement of electrons is called. To understand electrochemistry we need. Electrodes Concepts Chemistry.
From slidetodoc.com
Basic Concepts of Electrochemistry ELECTROCHEMISTRY Electricitydriven Electrodes Concepts Chemistry Interpret electrode potentials in terms of relative oxidant and reductant. The reaction can be started and stopped by connecting or disconnecting the two electrodes. Electrochemical cells allow measurement and control of a redox reaction. Describe and relate the definitions of electrode and cell potentials. Describe and relate the definitions of electrode and cell potentials. The electrode’s potential determines the analyte’s. Electrodes Concepts Chemistry.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field Electrodes Concepts Chemistry This movement of electrons is called. Electrochemistry is the study of chemical processes that cause electrons to move. The electrode’s potential determines the analyte’s form at the. Interpret electrode potentials in terms of relative oxidant and reductant strengths. An electrochemical cell consists of two electrically conductive electrodes immersed or in contact with an electrolyte (fig. To understand electrochemistry we need. Electrodes Concepts Chemistry.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12 Electrodes Concepts Chemistry Describe and relate the definitions of electrode and cell potentials. Electrochemistry is the study of chemical processes that cause electrons to move. To understand electrochemistry we need to appreciate five important and interrelated concepts: Describe and relate the definitions of electrode and cell potentials. The reaction can be started and stopped by connecting or disconnecting the two electrodes. Interpret electrode. Electrodes Concepts Chemistry.
From byjus.com
What is an active electrode? Explain with the help of an example Electrodes Concepts Chemistry Electrochemical cells allow measurement and control of a redox reaction. An electrochemical cell consists of two electrically conductive electrodes immersed or in contact with an electrolyte (fig. Describe and relate the definitions of electrode and cell potentials. The reaction can be started and stopped by connecting or disconnecting the two electrodes. Interpret electrode potentials in terms of relative oxidant and. Electrodes Concepts Chemistry.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry Electrodes Concepts Chemistry Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. To understand electrochemistry we need to appreciate five important and interrelated concepts: Describe and relate the definitions of electrode and cell potentials. Electrochemistry is the study of chemical processes that cause electrons to move. Describe and relate the definitions of. Electrodes Concepts Chemistry.