Liquid Nitrogen Acid Or Base . The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. It describes an acid as a molecule that will give away a proton — a type of subatomic particle, sometimes called a hydrogen ion — from one of its hydrogen atoms. The equation for ph is: The molecule, n2o2, absorbs light. Liquid nitrogen is a form of the element nitrogen that's cold enough to exist in a liquid state and is used for many cooling and cryogenic applications. The nitrogen in a fertilizer solution (measured in ppm n) has much more acid or base strength than the ph of that solution measured. Cooling a mixture of equal parts nitric oxide and nitrogen dioxide to −21 °c produces dinitrogen trioxide, a. Throughout history, chemists have created different definitions of acids and bases. Nitrogen is a component of proteins and of the genetic material (dna/rna) of all plants and animals.
from byjus.com
The equation for ph is: Cooling a mixture of equal parts nitric oxide and nitrogen dioxide to −21 °c produces dinitrogen trioxide, a. Throughout history, chemists have created different definitions of acids and bases. The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. Liquid nitrogen is a form of the element nitrogen that's cold enough to exist in a liquid state and is used for many cooling and cryogenic applications. The molecule, n2o2, absorbs light. The nitrogen in a fertilizer solution (measured in ppm n) has much more acid or base strength than the ph of that solution measured. It describes an acid as a molecule that will give away a proton — a type of subatomic particle, sometimes called a hydrogen ion — from one of its hydrogen atoms. Nitrogen is a component of proteins and of the genetic material (dna/rna) of all plants and animals.
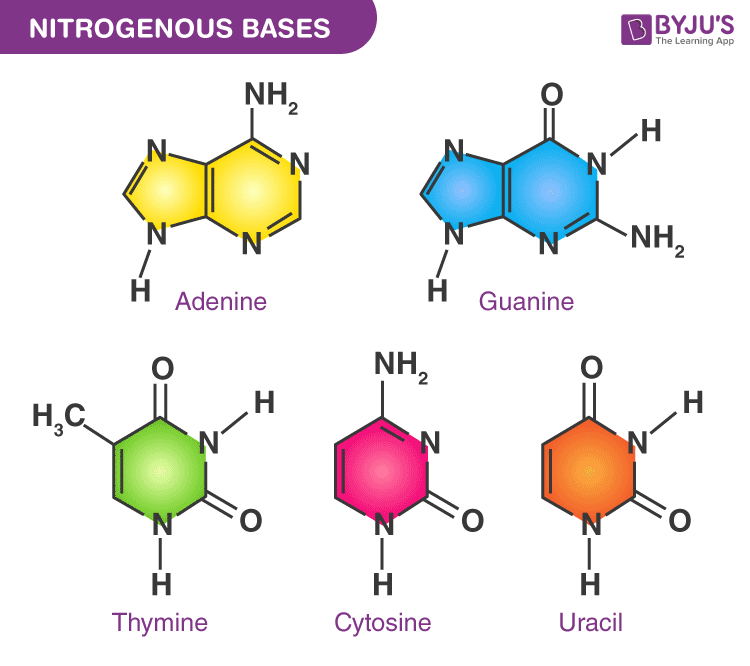
Nucleotide Structure, Examples and Function
Liquid Nitrogen Acid Or Base The nitrogen in a fertilizer solution (measured in ppm n) has much more acid or base strength than the ph of that solution measured. Nitrogen is a component of proteins and of the genetic material (dna/rna) of all plants and animals. The nitrogen in a fertilizer solution (measured in ppm n) has much more acid or base strength than the ph of that solution measured. The equation for ph is: It describes an acid as a molecule that will give away a proton — a type of subatomic particle, sometimes called a hydrogen ion — from one of its hydrogen atoms. The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. Liquid nitrogen is a form of the element nitrogen that's cold enough to exist in a liquid state and is used for many cooling and cryogenic applications. Cooling a mixture of equal parts nitric oxide and nitrogen dioxide to −21 °c produces dinitrogen trioxide, a. Throughout history, chemists have created different definitions of acids and bases. The molecule, n2o2, absorbs light.
From 1chemstaab.blogspot.com
Chemistry One Staab 201516 Acids and Bases Unit 8 Liquid Nitrogen Acid Or Base Liquid nitrogen is a form of the element nitrogen that's cold enough to exist in a liquid state and is used for many cooling and cryogenic applications. The molecule, n2o2, absorbs light. The nitrogen in a fertilizer solution (measured in ppm n) has much more acid or base strength than the ph of that solution measured. The numbers of the. Liquid Nitrogen Acid Or Base.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Liquid Nitrogen Acid Or Base The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. Nitrogen is a component of proteins and of the genetic material (dna/rna) of all plants and animals. Cooling a mixture of equal parts nitric oxide and nitrogen dioxide to −21 °c produces dinitrogen trioxide, a. The equation for ph is: The molecule, n2o2,. Liquid Nitrogen Acid Or Base.
From www.youtube.com
Nitric Acid What Happens When HNO3 acid is heated Liquid Nitrogen Acid Or Base The nitrogen in a fertilizer solution (measured in ppm n) has much more acid or base strength than the ph of that solution measured. The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. Liquid nitrogen is a form of the element nitrogen that's cold enough to exist in a liquid state and. Liquid Nitrogen Acid Or Base.
From www.youtube.com
How To Make Solid Nitrogen! (From Liquid Nitrogen) YouTube Liquid Nitrogen Acid Or Base The molecule, n2o2, absorbs light. Liquid nitrogen is a form of the element nitrogen that's cold enough to exist in a liquid state and is used for many cooling and cryogenic applications. Throughout history, chemists have created different definitions of acids and bases. It describes an acid as a molecule that will give away a proton — a type of. Liquid Nitrogen Acid Or Base.
From caloxinc.com
5 Facts about Liquid Nitrogen Liquid Nitrogen Acid Or Base It describes an acid as a molecule that will give away a proton — a type of subatomic particle, sometimes called a hydrogen ion — from one of its hydrogen atoms. The nitrogen in a fertilizer solution (measured in ppm n) has much more acid or base strength than the ph of that solution measured. The molecule, n2o2, absorbs light.. Liquid Nitrogen Acid Or Base.
From www.researchgate.net
Nitrogen phase state diagram Download Scientific Diagram Liquid Nitrogen Acid Or Base The molecule, n2o2, absorbs light. Cooling a mixture of equal parts nitric oxide and nitrogen dioxide to −21 °c produces dinitrogen trioxide, a. The nitrogen in a fertilizer solution (measured in ppm n) has much more acid or base strength than the ph of that solution measured. The equation for ph is: Throughout history, chemists have created different definitions of. Liquid Nitrogen Acid Or Base.
From melscience.com
Properties of liquid nitrogen. Laboratory experiments with liquid Liquid Nitrogen Acid Or Base The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. The nitrogen in a fertilizer solution (measured in ppm n) has much more acid or base strength than the ph of that solution measured. The equation for ph is: Liquid nitrogen is a form of the element nitrogen that's cold enough to exist. Liquid Nitrogen Acid Or Base.
From www.researchgate.net
The experimental setup using liquid nitrogen. Download Scientific Liquid Nitrogen Acid Or Base It describes an acid as a molecule that will give away a proton — a type of subatomic particle, sometimes called a hydrogen ion — from one of its hydrogen atoms. Throughout history, chemists have created different definitions of acids and bases. Nitrogen is a component of proteins and of the genetic material (dna/rna) of all plants and animals. The. Liquid Nitrogen Acid Or Base.
From pediaa.com
Difference Between Liquid Nitrogen and Nitrogen Gas Definition Liquid Nitrogen Acid Or Base It describes an acid as a molecule that will give away a proton — a type of subatomic particle, sometimes called a hydrogen ion — from one of its hydrogen atoms. Throughout history, chemists have created different definitions of acids and bases. Nitrogen is a component of proteins and of the genetic material (dna/rna) of all plants and animals. The. Liquid Nitrogen Acid Or Base.
From www.slideserve.com
PPT Nucleic Acids PowerPoint Presentation, free download ID1184338 Liquid Nitrogen Acid Or Base Throughout history, chemists have created different definitions of acids and bases. It describes an acid as a molecule that will give away a proton — a type of subatomic particle, sometimes called a hydrogen ion — from one of its hydrogen atoms. The nitrogen in a fertilizer solution (measured in ppm n) has much more acid or base strength than. Liquid Nitrogen Acid Or Base.
From kids.britannica.com
nitrogen Students Britannica Kids Homework Help Liquid Nitrogen Acid Or Base It describes an acid as a molecule that will give away a proton — a type of subatomic particle, sometimes called a hydrogen ion — from one of its hydrogen atoms. The nitrogen in a fertilizer solution (measured in ppm n) has much more acid or base strength than the ph of that solution measured. Throughout history, chemists have created. Liquid Nitrogen Acid Or Base.
From amchemistryblog.wordpress.com
4a. Acids and Bases Antonia's chemistry blog Liquid Nitrogen Acid Or Base The molecule, n2o2, absorbs light. Nitrogen is a component of proteins and of the genetic material (dna/rna) of all plants and animals. The nitrogen in a fertilizer solution (measured in ppm n) has much more acid or base strength than the ph of that solution measured. The equation for ph is: Throughout history, chemists have created different definitions of acids. Liquid Nitrogen Acid Or Base.
From alevelchemistry.co.uk
Acids Facts, Summary, Weak & Strong ALevel Chemistry Revision Liquid Nitrogen Acid Or Base The nitrogen in a fertilizer solution (measured in ppm n) has much more acid or base strength than the ph of that solution measured. Liquid nitrogen is a form of the element nitrogen that's cold enough to exist in a liquid state and is used for many cooling and cryogenic applications. The numbers of the ph scale come from a. Liquid Nitrogen Acid Or Base.
From pixels.com
Liquid Nitrogen Photograph by Andrew Mcclenaghan/science Photo Library. Liquid Nitrogen Acid Or Base Throughout history, chemists have created different definitions of acids and bases. Liquid nitrogen is a form of the element nitrogen that's cold enough to exist in a liquid state and is used for many cooling and cryogenic applications. It describes an acid as a molecule that will give away a proton — a type of subatomic particle, sometimes called a. Liquid Nitrogen Acid Or Base.
From fphoto.photoshelter.com
science element nitrogen Fundamental Photographs The Art of Science Liquid Nitrogen Acid Or Base Throughout history, chemists have created different definitions of acids and bases. The molecule, n2o2, absorbs light. The equation for ph is: The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. It describes an acid as a molecule that will give away a proton — a type of subatomic particle, sometimes called a. Liquid Nitrogen Acid Or Base.
From byjus.com
Biomolecules Important Notes for NEET Biology Liquid Nitrogen Acid Or Base The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. Liquid nitrogen is a form of the element nitrogen that's cold enough to exist in a liquid state and is used for many cooling and cryogenic applications. The molecule, n2o2, absorbs light. Nitrogen is a component of proteins and of the genetic material. Liquid Nitrogen Acid Or Base.
From www.chemistrylearner.com
Nitrogen Facts, Symbol, Discovery, Properties, Uses Liquid Nitrogen Acid Or Base Nitrogen is a component of proteins and of the genetic material (dna/rna) of all plants and animals. Cooling a mixture of equal parts nitric oxide and nitrogen dioxide to −21 °c produces dinitrogen trioxide, a. Liquid nitrogen is a form of the element nitrogen that's cold enough to exist in a liquid state and is used for many cooling and. Liquid Nitrogen Acid Or Base.
From www.researchgate.net
1 Properties of liquid nitrogen used in model. Liquid nitrogen[80 Liquid Nitrogen Acid Or Base Cooling a mixture of equal parts nitric oxide and nitrogen dioxide to −21 °c produces dinitrogen trioxide, a. It describes an acid as a molecule that will give away a proton — a type of subatomic particle, sometimes called a hydrogen ion — from one of its hydrogen atoms. The nitrogen in a fertilizer solution (measured in ppm n) has. Liquid Nitrogen Acid Or Base.
From www.funnyjunk.com
Liquid Nitrogen Liquid Nitrogen Acid Or Base It describes an acid as a molecule that will give away a proton — a type of subatomic particle, sometimes called a hydrogen ion — from one of its hydrogen atoms. Nitrogen is a component of proteins and of the genetic material (dna/rna) of all plants and animals. The molecule, n2o2, absorbs light. Throughout history, chemists have created different definitions. Liquid Nitrogen Acid Or Base.
From www.youtube.com
Simple Feats of Science Liquid Nitrogen Experiments! YouTube Liquid Nitrogen Acid Or Base The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. The nitrogen in a fertilizer solution (measured in ppm n) has much more acid or base strength than the ph of that solution measured. It describes an acid as a molecule that will give away a proton — a type of subatomic particle,. Liquid Nitrogen Acid Or Base.
From www.slideserve.com
PPT Liquid Nitrogen PowerPoint Presentation, free download ID6193757 Liquid Nitrogen Acid Or Base The nitrogen in a fertilizer solution (measured in ppm n) has much more acid or base strength than the ph of that solution measured. It describes an acid as a molecule that will give away a proton — a type of subatomic particle, sometimes called a hydrogen ion — from one of its hydrogen atoms. Liquid nitrogen is a form. Liquid Nitrogen Acid Or Base.
From www.thoughtco.com
Liquid Nitrogen Facts, Safety and Uses Liquid Nitrogen Acid Or Base Liquid nitrogen is a form of the element nitrogen that's cold enough to exist in a liquid state and is used for many cooling and cryogenic applications. The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. Throughout history, chemists have created different definitions of acids and bases. The molecule, n2o2, absorbs light.. Liquid Nitrogen Acid Or Base.
From microbenotes.com
Watson and Crick DNA Model Molecular Biology / Microbe Notes Liquid Nitrogen Acid Or Base Cooling a mixture of equal parts nitric oxide and nitrogen dioxide to −21 °c produces dinitrogen trioxide, a. The nitrogen in a fertilizer solution (measured in ppm n) has much more acid or base strength than the ph of that solution measured. The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. The. Liquid Nitrogen Acid Or Base.
From www.prohealthsite.com
How Liquid Nitrogen is Used in the Medical Industry Liquid Nitrogen Acid Or Base Throughout history, chemists have created different definitions of acids and bases. Liquid nitrogen is a form of the element nitrogen that's cold enough to exist in a liquid state and is used for many cooling and cryogenic applications. The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. It describes an acid as. Liquid Nitrogen Acid Or Base.
From byjus.com
Nucleotide Structure, Examples and Function Liquid Nitrogen Acid Or Base Nitrogen is a component of proteins and of the genetic material (dna/rna) of all plants and animals. It describes an acid as a molecule that will give away a proton — a type of subatomic particle, sometimes called a hydrogen ion — from one of its hydrogen atoms. The numbers of the ph scale come from a formula for the. Liquid Nitrogen Acid Or Base.
From periodictable.com
Liquid nitrogen, a sample of the element Nitrogen in the Periodic Table Liquid Nitrogen Acid Or Base Cooling a mixture of equal parts nitric oxide and nitrogen dioxide to −21 °c produces dinitrogen trioxide, a. The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. Nitrogen is a component of proteins and of the genetic material (dna/rna) of all plants and animals. The molecule, n2o2, absorbs light. It describes an. Liquid Nitrogen Acid Or Base.
From nadia-blogfields.blogspot.com
Boiling Point of Nitrogen Liquid Nitrogen Acid Or Base Cooling a mixture of equal parts nitric oxide and nitrogen dioxide to −21 °c produces dinitrogen trioxide, a. The equation for ph is: The molecule, n2o2, absorbs light. Liquid nitrogen is a form of the element nitrogen that's cold enough to exist in a liquid state and is used for many cooling and cryogenic applications. The nitrogen in a fertilizer. Liquid Nitrogen Acid Or Base.
From www.toppr.com
What species are characteristic of acid and of bases in the following Liquid Nitrogen Acid Or Base Throughout history, chemists have created different definitions of acids and bases. The molecule, n2o2, absorbs light. The nitrogen in a fertilizer solution (measured in ppm n) has much more acid or base strength than the ph of that solution measured. Cooling a mixture of equal parts nitric oxide and nitrogen dioxide to −21 °c produces dinitrogen trioxide, a. Liquid nitrogen. Liquid Nitrogen Acid Or Base.
From www.slideserve.com
PPT Liquid Nitrogen Safety PowerPoint Presentation, free download Liquid Nitrogen Acid Or Base Cooling a mixture of equal parts nitric oxide and nitrogen dioxide to −21 °c produces dinitrogen trioxide, a. Nitrogen is a component of proteins and of the genetic material (dna/rna) of all plants and animals. Throughout history, chemists have created different definitions of acids and bases. The nitrogen in a fertilizer solution (measured in ppm n) has much more acid. Liquid Nitrogen Acid Or Base.
From techiescientist.com
16 Uses of Nitrogen That You Must Know Techiescientist Liquid Nitrogen Acid Or Base Liquid nitrogen is a form of the element nitrogen that's cold enough to exist in a liquid state and is used for many cooling and cryogenic applications. The nitrogen in a fertilizer solution (measured in ppm n) has much more acid or base strength than the ph of that solution measured. Nitrogen is a component of proteins and of the. Liquid Nitrogen Acid Or Base.
From www.slideserve.com
PPT Liquid Nitrogen PowerPoint Presentation, free download ID6193757 Liquid Nitrogen Acid Or Base The molecule, n2o2, absorbs light. Liquid nitrogen is a form of the element nitrogen that's cold enough to exist in a liquid state and is used for many cooling and cryogenic applications. Nitrogen is a component of proteins and of the genetic material (dna/rna) of all plants and animals. Cooling a mixture of equal parts nitric oxide and nitrogen dioxide. Liquid Nitrogen Acid Or Base.
From guitarscalechart.z28.web.core.windows.net
acid and base ph scale chart Show me the ph scale Liquid Nitrogen Acid Or Base The molecule, n2o2, absorbs light. The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. The nitrogen in a fertilizer solution (measured in ppm n) has much more acid or base strength than the ph of that solution measured. Liquid nitrogen is a form of the element nitrogen that's cold enough to exist. Liquid Nitrogen Acid Or Base.
From kalstein.co.nz
What are the advantages and disadvantages of liquid nitrogen? Kalstein Liquid Nitrogen Acid Or Base The molecule, n2o2, absorbs light. The nitrogen in a fertilizer solution (measured in ppm n) has much more acid or base strength than the ph of that solution measured. The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. Cooling a mixture of equal parts nitric oxide and nitrogen dioxide to −21 °c. Liquid Nitrogen Acid Or Base.
From pediaa.com
Difference Between Liquid Nitrogen and Nitrogen Gas Definition Liquid Nitrogen Acid Or Base Liquid nitrogen is a form of the element nitrogen that's cold enough to exist in a liquid state and is used for many cooling and cryogenic applications. The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. Cooling a mixture of equal parts nitric oxide and nitrogen dioxide to −21 °c produces dinitrogen. Liquid Nitrogen Acid Or Base.