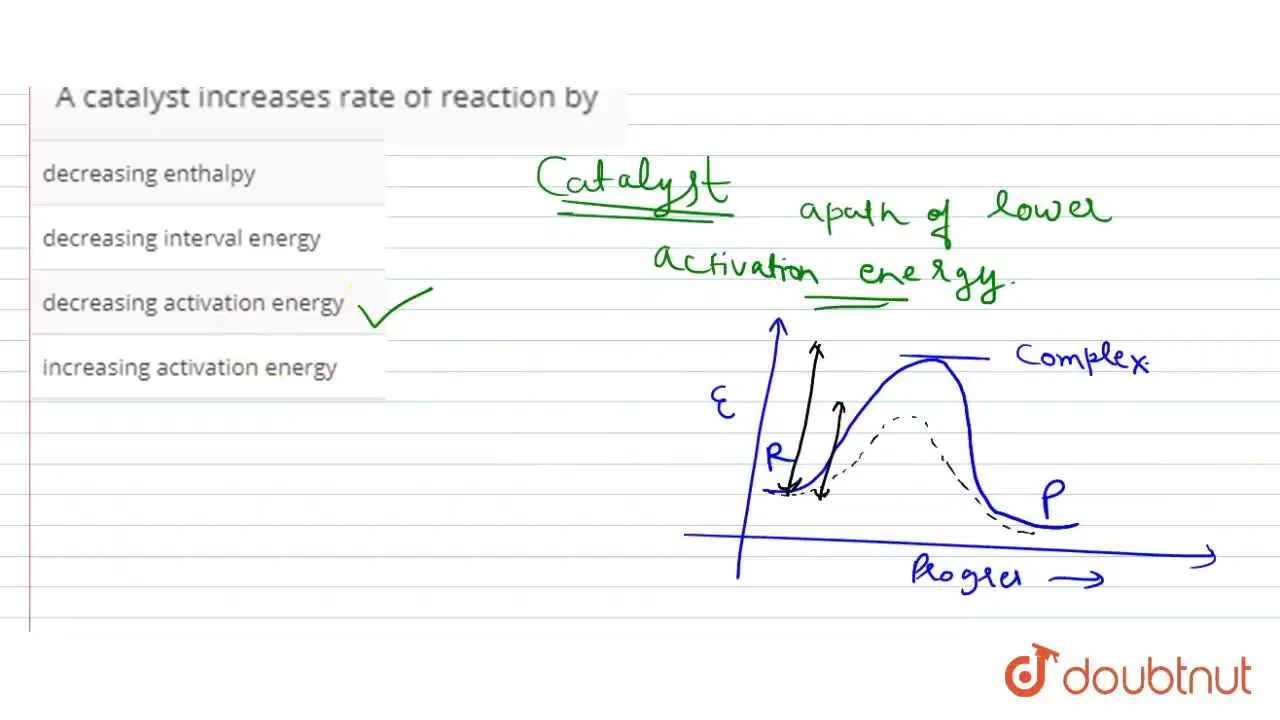
A Positive Catalyst Increases The Rate Of Reaction By . A catalyst increases the rate of reaction in both forward and backward directions by providing an alternate pathway with lower activation energy. A positive catalyst lowers the activation energy of a reaction and speeds up its rate. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. This page explains how adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction. In contrast, a negative catalyst makes a reaction less favorable and slows its rate. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst is a chemical substance. If the activation energy is reduced,.
from www.doubtnut.com
If the activation energy is reduced,. It assumes that you are already familiar with basic ideas about the collision theory of reaction. A catalyst is a chemical substance. This page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. A positive catalyst lowers the activation energy of a reaction and speeds up its rate. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst increases the rate of reaction in both forward and backward directions by providing an alternate pathway with lower activation energy. In contrast, a negative catalyst makes a reaction less favorable and slows its rate. This page explains how adding a catalyst affects the rate of a reaction.
A catalyst increases rate of reaction by
A Positive Catalyst Increases The Rate Of Reaction By It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. This page explains how adding a catalyst affects the rate of a reaction. In contrast, a negative catalyst makes a reaction less favorable and slows its rate. A catalyst increases the rate of reaction in both forward and backward directions by providing an alternate pathway with lower activation energy. If the activation energy is reduced,. It assumes that you are already familiar with basic ideas about the collision theory of reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst is a chemical substance. A positive catalyst lowers the activation energy of a reaction and speeds up its rate. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube A Positive Catalyst Increases The Rate Of Reaction By A catalyst increases the rate of reaction in both forward and backward directions by providing an alternate pathway with lower activation energy. This page explains how adding a catalyst affects the rate of a reaction. If the activation energy is reduced,. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. It. A Positive Catalyst Increases The Rate Of Reaction By.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst A Positive Catalyst Increases The Rate Of Reaction By A positive catalyst lowers the activation energy of a reaction and speeds up its rate. In contrast, a negative catalyst makes a reaction less favorable and slows its rate. If the activation energy is reduced,. A catalyst is a chemical substance. It assumes that you are already familiar with basic ideas about the collision theory of reaction. It assumes familiarity. A Positive Catalyst Increases The Rate Of Reaction By.
From helecu.com
Factors Affecting Reaction Rates Chemistry Atoms First (2022) A Positive Catalyst Increases The Rate Of Reaction By If the activation energy is reduced,. This page explains how adding a catalyst affects the rate of a reaction. A positive catalyst lowers the activation energy of a reaction and speeds up its rate. In contrast, a negative catalyst makes a reaction less favorable and slows its rate. A catalyst increases the rate of reaction in both forward and backward. A Positive Catalyst Increases The Rate Of Reaction By.
From slideplayer.com
Catalysts Rates of Reactions. ppt download A Positive Catalyst Increases The Rate Of Reaction By In contrast, a negative catalyst makes a reaction less favorable and slows its rate. A positive catalyst lowers the activation energy of a reaction and speeds up its rate. This page explains how adding a catalyst affects the rate of a reaction. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst.. A Positive Catalyst Increases The Rate Of Reaction By.
From www.numerade.com
SOLVEDA catalyst increases rate of reaction by (a) decreasing enthalpy A Positive Catalyst Increases The Rate Of Reaction By This page explains how adding a catalyst affects the rate of a reaction. If the activation energy is reduced,. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. It assumes that you are already familiar with basic ideas about the collision theory of reaction. This page describes and explains the way. A Positive Catalyst Increases The Rate Of Reaction By.
From www.doubtnut.com
When a catalyst increases the rate of chemical reaction the rate const A Positive Catalyst Increases The Rate Of Reaction By A catalyst is a chemical substance. It assumes that you are already familiar with basic ideas about the collision theory of reaction. If the activation energy is reduced,. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst increases the rate of reaction in both forward and backward directions by. A Positive Catalyst Increases The Rate Of Reaction By.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii A Positive Catalyst Increases The Rate Of Reaction By If the activation energy is reduced,. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst increases the rate of reaction in both forward and backward directions by providing an alternate pathway with. A Positive Catalyst Increases The Rate Of Reaction By.
From www.nagwa.com
Question Video Recalling the Practical Changes That Can Be Made to A Positive Catalyst Increases The Rate Of Reaction By In contrast, a negative catalyst makes a reaction less favorable and slows its rate. This page explains how adding a catalyst affects the rate of a reaction. If the activation energy is reduced,. A positive catalyst lowers the activation energy of a reaction and speeds up its rate. Catalysis is the process that alters the rate of a chemical reaction. A Positive Catalyst Increases The Rate Of Reaction By.
From ppt-online.org
Rates of reaction презентация онлайн A Positive Catalyst Increases The Rate Of Reaction By If the activation energy is reduced,. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. A catalyst increases the rate of reaction in both forward and backward directions by providing an alternate pathway with lower activation energy. In contrast, a negative catalyst makes a reaction less favorable and slows its rate. This. A Positive Catalyst Increases The Rate Of Reaction By.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry A Positive Catalyst Increases The Rate Of Reaction By If the activation energy is reduced,. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A positive catalyst lowers the activation energy of a reaction and speeds up its rate. A catalyst is a. A Positive Catalyst Increases The Rate Of Reaction By.
From www.slideserve.com
PPT Factors Affecting the Rate of a Chemical Reaction PowerPoint A Positive Catalyst Increases The Rate Of Reaction By This page explains how adding a catalyst affects the rate of a reaction. A catalyst is a chemical substance. A positive catalyst lowers the activation energy of a reaction and speeds up its rate. It assumes that you are already familiar with basic ideas about the collision theory of reaction. Catalysis is the process that alters the rate of a. A Positive Catalyst Increases The Rate Of Reaction By.
From byjus.com
Which of the following statement is incorrect about positive and A Positive Catalyst Increases The Rate Of Reaction By Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. If the activation energy is reduced,. This page explains how adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction. A catalyst is a chemical substance. It. A Positive Catalyst Increases The Rate Of Reaction By.
From byjus.com
Reaction Coordinate Diagram An Overview of Reaction Coordinate A Positive Catalyst Increases The Rate Of Reaction By In contrast, a negative catalyst makes a reaction less favorable and slows its rate. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst increases the rate of reaction in both forward and backward directions by providing an alternate pathway with lower activation energy. It assumes that you are already familiar. A Positive Catalyst Increases The Rate Of Reaction By.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation A Positive Catalyst Increases The Rate Of Reaction By In contrast, a negative catalyst makes a reaction less favorable and slows its rate. This page explains how adding a catalyst affects the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the. A Positive Catalyst Increases The Rate Of Reaction By.
From byjus.com
How does catalyst affect activation energy? A Positive Catalyst Increases The Rate Of Reaction By A positive catalyst lowers the activation energy of a reaction and speeds up its rate. A catalyst is a chemical substance. A catalyst increases the rate of reaction in both forward and backward directions by providing an alternate pathway with lower activation energy. In contrast, a negative catalyst makes a reaction less favorable and slows its rate. Catalysis is the. A Positive Catalyst Increases The Rate Of Reaction By.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis A Positive Catalyst Increases The Rate Of Reaction By Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. If the activation energy is reduced,. This page explains how adding a catalyst affects the rate of a reaction. A positive catalyst lowers the activation energy of a reaction and speeds up its rate. In contrast, a negative catalyst makes a reaction. A Positive Catalyst Increases The Rate Of Reaction By.
From socratic.org
What will occur if a catalyst is added to a reaction mixture? Socratic A Positive Catalyst Increases The Rate Of Reaction By Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is a chemical substance. It assumes that you are already familiar with basic ideas about the collision theory of reaction. This page explains how adding a catalyst affects the rate of a reaction. This page describes and explains the way. A Positive Catalyst Increases The Rate Of Reaction By.
From slideplayer.com
CHEMICAL KINITICS Kononova T.O.. ppt download A Positive Catalyst Increases The Rate Of Reaction By A positive catalyst lowers the activation energy of a reaction and speeds up its rate. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. A catalyst is a chemical substance. In contrast, a negative catalyst makes a reaction less favorable and slows its rate. Catalysis is the process that alters the rate. A Positive Catalyst Increases The Rate Of Reaction By.
From byjus.com
In a given reaction A > 2B. As time proceeds which of the following is A Positive Catalyst Increases The Rate Of Reaction By In contrast, a negative catalyst makes a reaction less favorable and slows its rate. A positive catalyst lowers the activation energy of a reaction and speeds up its rate. If the activation energy is reduced,. This page explains how adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision theory of reaction. A Positive Catalyst Increases The Rate Of Reaction By.
From slideplayer.com
THE RATES OF REACTIONS Chapter 13. Reaction Rate The reaction rate is A Positive Catalyst Increases The Rate Of Reaction By It assumes that you are already familiar with basic ideas about the collision theory of reaction. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. This page explains how adding a catalyst affects the rate of a reaction. In contrast, a negative catalyst makes a reaction less favorable and slows its rate.. A Positive Catalyst Increases The Rate Of Reaction By.
From byjus.com
Choose the graph which correctly represents the reaction path when a A Positive Catalyst Increases The Rate Of Reaction By It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. A positive catalyst lowers the activation energy of a reaction and speeds up its rate. This page describes and explains the way that adding a catalyst affects the rate of a reaction. If the activation energy is reduced,. This page explains how adding. A Positive Catalyst Increases The Rate Of Reaction By.
From www.doubtnut.com
A catalyst increases rate of reaction by A Positive Catalyst Increases The Rate Of Reaction By This page explains how adding a catalyst affects the rate of a reaction. A catalyst increases the rate of reaction in both forward and backward directions by providing an alternate pathway with lower activation energy. A positive catalyst lowers the activation energy of a reaction and speeds up its rate. In contrast, a negative catalyst makes a reaction less favorable. A Positive Catalyst Increases The Rate Of Reaction By.
From www.numerade.com
SOLVED Which will increase the rate of a chemical reaction? A. Adding A Positive Catalyst Increases The Rate Of Reaction By A catalyst increases the rate of reaction in both forward and backward directions by providing an alternate pathway with lower activation energy. This page explains how adding a catalyst affects the rate of a reaction. A catalyst is a chemical substance. If the activation energy is reduced,. This page describes and explains the way that adding a catalyst affects the. A Positive Catalyst Increases The Rate Of Reaction By.
From www.numerade.com
SOLVED Draw a reaction coordinate diagram for the reversible reaction A Positive Catalyst Increases The Rate Of Reaction By This page explains how adding a catalyst affects the rate of a reaction. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst increases the rate of reaction in both forward and backward directions by providing an alternate pathway with lower activation energy. A catalyst is a chemical substance. If. A Positive Catalyst Increases The Rate Of Reaction By.
From exoqpbamm.blob.core.windows.net
A Catalyst Increases The Rate Of A Reaction By Providing A Path That at A Positive Catalyst Increases The Rate Of Reaction By This page explains how adding a catalyst affects the rate of a reaction. A catalyst increases the rate of reaction in both forward and backward directions by providing an alternate pathway with lower activation energy. If the activation energy is reduced,. A catalyst is a chemical substance. It assumes familiarity with basic concepts in the collision theory of reaction rates,. A Positive Catalyst Increases The Rate Of Reaction By.
From answerhappy.com
A catalyst is a substance that increases the rate of reaction but does A Positive Catalyst Increases The Rate Of Reaction By A positive catalyst lowers the activation energy of a reaction and speeds up its rate. A catalyst is a chemical substance. A catalyst increases the rate of reaction in both forward and backward directions by providing an alternate pathway with lower activation energy. In contrast, a negative catalyst makes a reaction less favorable and slows its rate. Catalysis is the. A Positive Catalyst Increases The Rate Of Reaction By.
From ar.inspiredpencil.com
Catalyst Chemical Reaction A Positive Catalyst Increases The Rate Of Reaction By If the activation energy is reduced,. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst increases the rate of reaction in both forward and backward directions by providing an alternate pathway with lower activation energy. It assumes that you are already familiar with basic ideas about the collision theory of. A Positive Catalyst Increases The Rate Of Reaction By.
From www.nagwa.com
Question Video Determining How a Catalyst Affects the Energy Profile A Positive Catalyst Increases The Rate Of Reaction By It assumes that you are already familiar with basic ideas about the collision theory of reaction. A catalyst is a chemical substance. This page explains how adding a catalyst affects the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst increases the rate of reaction in. A Positive Catalyst Increases The Rate Of Reaction By.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions A Positive Catalyst Increases The Rate Of Reaction By If the activation energy is reduced,. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. It assumes that you are already familiar with basic ideas about the collision theory of reaction. This page describes. A Positive Catalyst Increases The Rate Of Reaction By.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst A Positive Catalyst Increases The Rate Of Reaction By If the activation energy is reduced,. It assumes that you are already familiar with basic ideas about the collision theory of reaction. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. A catalyst is a chemical substance. This page describes and explains the way that adding a catalyst affects the rate of. A Positive Catalyst Increases The Rate Of Reaction By.
From www.edukate.me
Explain The Effect of Catalyst on the Rate of Reaction A catalyst A Positive Catalyst Increases The Rate Of Reaction By This page explains how adding a catalyst affects the rate of a reaction. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A positive catalyst lowers the activation energy of a reaction and speeds. A Positive Catalyst Increases The Rate Of Reaction By.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction A Positive Catalyst Increases The Rate Of Reaction By A positive catalyst lowers the activation energy of a reaction and speeds up its rate. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst is a chemical substance. In contrast, a negative catalyst makes a reaction less favorable and slows its rate. This page explains how adding a catalyst affects. A Positive Catalyst Increases The Rate Of Reaction By.
From www.expii.com
Rate of Reaction (Enzymes) — Role & Importance Expii A Positive Catalyst Increases The Rate Of Reaction By It assumes that you are already familiar with basic ideas about the collision theory of reaction. A catalyst is a chemical substance. A positive catalyst lowers the activation energy of a reaction and speeds up its rate. This page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts. A Positive Catalyst Increases The Rate Of Reaction By.
From infinitylearn.com
A catalyst increases the rate of reaction by Sri Chaitanya Infinity A Positive Catalyst Increases The Rate Of Reaction By If the activation energy is reduced,. A positive catalyst lowers the activation energy of a reaction and speeds up its rate. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Catalysis is the process that. A Positive Catalyst Increases The Rate Of Reaction By.
From brainly.com
A catalyst increases the reaction rate of a reaction by (3 points A Positive Catalyst Increases The Rate Of Reaction By Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst increases the rate of reaction in both forward and backward directions by providing an alternate pathway with lower activation energy. A catalyst is. A Positive Catalyst Increases The Rate Of Reaction By.