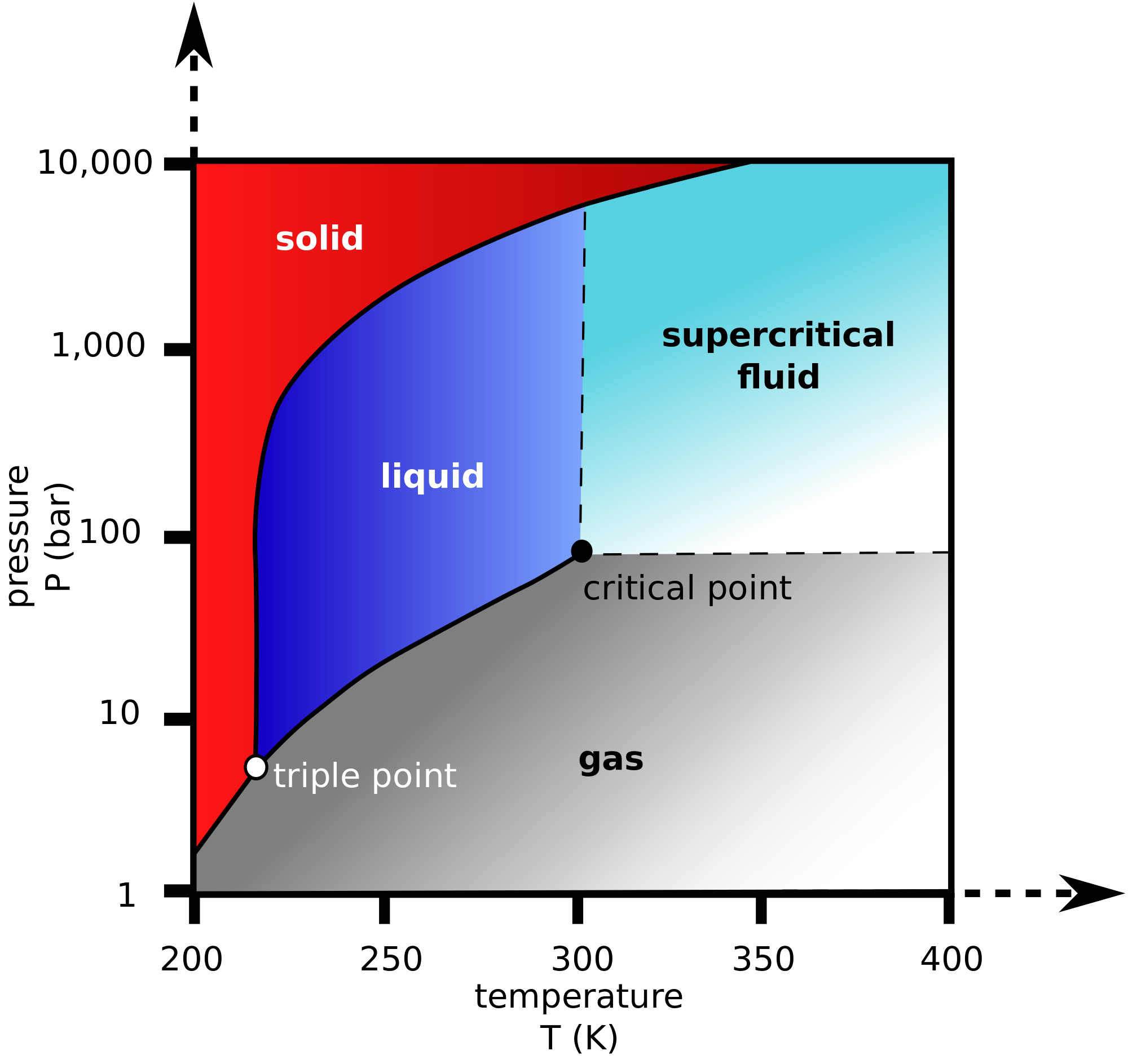
Does Boiling Temperature Increase With Pressure . The boiling point of something is the point where the vapour pressure equals or exceeds the atmospheric pressure, as stated. Therefore, increasing the pressure displaces the equilibrium (c.f. When the vapour pressure equals atmospheric. As temperature increases, more particles have enough energy to escape to the gas phase. The boiling point of water at atmospheric pressure (101315 pa) is 100c, however the boiling point is changing with. For example, water will remain at 100ºc (at a pressure of 1 atm or. When a gas, the molecules occupy a far greater volume than they do when a liquid. This increases the vapour pressure. Therefore the temperature of the liquid remains constant during boiling. The boiling point of water depends. The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to atmospheric pressure. 95 rows water boiling point in pressure higher than atm.
from socratic.org
This increases the vapour pressure. Therefore, increasing the pressure displaces the equilibrium (c.f. As temperature increases, more particles have enough energy to escape to the gas phase. The boiling point of water at atmospheric pressure (101315 pa) is 100c, however the boiling point is changing with. The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to atmospheric pressure. When a gas, the molecules occupy a far greater volume than they do when a liquid. When the vapour pressure equals atmospheric. The boiling point of something is the point where the vapour pressure equals or exceeds the atmospheric pressure, as stated. Therefore the temperature of the liquid remains constant during boiling. For example, water will remain at 100ºc (at a pressure of 1 atm or.
What is the relation between critical temperature and boiling point or
Does Boiling Temperature Increase With Pressure As temperature increases, more particles have enough energy to escape to the gas phase. Therefore the temperature of the liquid remains constant during boiling. Therefore, increasing the pressure displaces the equilibrium (c.f. 95 rows water boiling point in pressure higher than atm. The boiling point of something is the point where the vapour pressure equals or exceeds the atmospheric pressure, as stated. This increases the vapour pressure. For example, water will remain at 100ºc (at a pressure of 1 atm or. The boiling point of water depends. When a gas, the molecules occupy a far greater volume than they do when a liquid. When the vapour pressure equals atmospheric. The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to atmospheric pressure. The boiling point of water at atmospheric pressure (101315 pa) is 100c, however the boiling point is changing with. As temperature increases, more particles have enough energy to escape to the gas phase.
From www.teachoo.com
What is the Difference between Evaporation and Boiling? Class 9 Does Boiling Temperature Increase With Pressure The boiling point of something is the point where the vapour pressure equals or exceeds the atmospheric pressure, as stated. The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to atmospheric pressure. This increases the vapour pressure. Therefore, increasing the pressure displaces the equilibrium (c.f. When a gas, the molecules occupy a. Does Boiling Temperature Increase With Pressure.
From hxeuuhbcr.blob.core.windows.net
Does Vapor Pressure Increase Boiling at Dennis blog Does Boiling Temperature Increase With Pressure When the vapour pressure equals atmospheric. The boiling point of water at atmospheric pressure (101315 pa) is 100c, however the boiling point is changing with. The boiling point of something is the point where the vapour pressure equals or exceeds the atmospheric pressure, as stated. The boiling point of water depends. As temperature increases, more particles have enough energy to. Does Boiling Temperature Increase With Pressure.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Does Boiling Temperature Increase With Pressure The boiling point of water depends. The boiling point of water at atmospheric pressure (101315 pa) is 100c, however the boiling point is changing with. The boiling point of something is the point where the vapour pressure equals or exceeds the atmospheric pressure, as stated. When the vapour pressure equals atmospheric. This increases the vapour pressure. 95 rows water boiling. Does Boiling Temperature Increase With Pressure.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Does Boiling Temperature Increase With Pressure 95 rows water boiling point in pressure higher than atm. When a gas, the molecules occupy a far greater volume than they do when a liquid. Therefore, increasing the pressure displaces the equilibrium (c.f. When the vapour pressure equals atmospheric. This increases the vapour pressure. The boiling point of something is the point where the vapour pressure equals or exceeds. Does Boiling Temperature Increase With Pressure.
From www.ck12.org
Heating and Cooling Curves CK12 Foundation Does Boiling Temperature Increase With Pressure This increases the vapour pressure. For example, water will remain at 100ºc (at a pressure of 1 atm or. Therefore, increasing the pressure displaces the equilibrium (c.f. The boiling point of something is the point where the vapour pressure equals or exceeds the atmospheric pressure, as stated. 95 rows water boiling point in pressure higher than atm. The boiling point. Does Boiling Temperature Increase With Pressure.
From socratic.org
What is the relation between critical temperature and boiling point or Does Boiling Temperature Increase With Pressure Therefore the temperature of the liquid remains constant during boiling. The boiling point of water depends. The boiling point of something is the point where the vapour pressure equals or exceeds the atmospheric pressure, as stated. Therefore, increasing the pressure displaces the equilibrium (c.f. For example, water will remain at 100ºc (at a pressure of 1 atm or. The boiling. Does Boiling Temperature Increase With Pressure.
From eatingrules.com
The Science of Pressure Cookers Eating Rules Does Boiling Temperature Increase With Pressure 95 rows water boiling point in pressure higher than atm. When the vapour pressure equals atmospheric. As temperature increases, more particles have enough energy to escape to the gas phase. The boiling point of something is the point where the vapour pressure equals or exceeds the atmospheric pressure, as stated. For example, water will remain at 100ºc (at a pressure. Does Boiling Temperature Increase With Pressure.
From sciencenotes.org
How to Boil Water at Room Temperature Does Boiling Temperature Increase With Pressure Therefore, increasing the pressure displaces the equilibrium (c.f. When the vapour pressure equals atmospheric. The boiling point of water at atmospheric pressure (101315 pa) is 100c, however the boiling point is changing with. 95 rows water boiling point in pressure higher than atm. This increases the vapour pressure. The boiling point of water depends. As temperature increases, more particles have. Does Boiling Temperature Increase With Pressure.
From www.scienceabc.com
Why Does Water Boil Quickly At High Altitudes? » ScienceABC Does Boiling Temperature Increase With Pressure Therefore the temperature of the liquid remains constant during boiling. Therefore, increasing the pressure displaces the equilibrium (c.f. 95 rows water boiling point in pressure higher than atm. When a gas, the molecules occupy a far greater volume than they do when a liquid. When the vapour pressure equals atmospheric. The boiling point of something is the point where the. Does Boiling Temperature Increase With Pressure.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation ID705859 Does Boiling Temperature Increase With Pressure As temperature increases, more particles have enough energy to escape to the gas phase. 95 rows water boiling point in pressure higher than atm. The boiling point of something is the point where the vapour pressure equals or exceeds the atmospheric pressure, as stated. Therefore, increasing the pressure displaces the equilibrium (c.f. The boiling point of water depends. When a. Does Boiling Temperature Increase With Pressure.
From mungfali.com
Water Boiling Point Pressure Chart Does Boiling Temperature Increase With Pressure The boiling point of water at atmospheric pressure (101315 pa) is 100c, however the boiling point is changing with. Therefore, increasing the pressure displaces the equilibrium (c.f. For example, water will remain at 100ºc (at a pressure of 1 atm or. When the vapour pressure equals atmospheric. As temperature increases, more particles have enough energy to escape to the gas. Does Boiling Temperature Increase With Pressure.
From facts.net
20 Fascinating Facts About Boiling Point Does Boiling Temperature Increase With Pressure Therefore the temperature of the liquid remains constant during boiling. The boiling point of water depends. As temperature increases, more particles have enough energy to escape to the gas phase. This increases the vapour pressure. The boiling point of something is the point where the vapour pressure equals or exceeds the atmospheric pressure, as stated. Therefore, increasing the pressure displaces. Does Boiling Temperature Increase With Pressure.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube Does Boiling Temperature Increase With Pressure The boiling point of water depends. The boiling point of something is the point where the vapour pressure equals or exceeds the atmospheric pressure, as stated. The boiling point of water at atmospheric pressure (101315 pa) is 100c, however the boiling point is changing with. When the vapour pressure equals atmospheric. When a gas, the molecules occupy a far greater. Does Boiling Temperature Increase With Pressure.
From med.libretexts.org
2.3 Gaseous Exchange Mechanism Medicine LibreTexts Does Boiling Temperature Increase With Pressure The boiling point of something is the point where the vapour pressure equals or exceeds the atmospheric pressure, as stated. Therefore, increasing the pressure displaces the equilibrium (c.f. As temperature increases, more particles have enough energy to escape to the gas phase. When a gas, the molecules occupy a far greater volume than they do when a liquid. For example,. Does Boiling Temperature Increase With Pressure.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Does Boiling Temperature Increase With Pressure The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to atmospheric pressure. For example, water will remain at 100ºc (at a pressure of 1 atm or. 95 rows water boiling point in pressure higher than atm. Therefore, increasing the pressure displaces the equilibrium (c.f. Therefore the temperature of the liquid remains constant. Does Boiling Temperature Increase With Pressure.
From guides.hostos.cuny.edu
Chapter 3 Solids and Liquids CHE 110 Introduction to Chemistry Does Boiling Temperature Increase With Pressure 95 rows water boiling point in pressure higher than atm. Therefore the temperature of the liquid remains constant during boiling. When a gas, the molecules occupy a far greater volume than they do when a liquid. The boiling point of something is the point where the vapour pressure equals or exceeds the atmospheric pressure, as stated. For example, water will. Does Boiling Temperature Increase With Pressure.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER Does Boiling Temperature Increase With Pressure Therefore, increasing the pressure displaces the equilibrium (c.f. When a gas, the molecules occupy a far greater volume than they do when a liquid. For example, water will remain at 100ºc (at a pressure of 1 atm or. This increases the vapour pressure. The boiling point of water at atmospheric pressure (101315 pa) is 100c, however the boiling point is. Does Boiling Temperature Increase With Pressure.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition Does Boiling Temperature Increase With Pressure Therefore, increasing the pressure displaces the equilibrium (c.f. 95 rows water boiling point in pressure higher than atm. As temperature increases, more particles have enough energy to escape to the gas phase. The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to atmospheric pressure. When the vapour pressure equals atmospheric. The boiling. Does Boiling Temperature Increase With Pressure.
From byjus.com
How does external pressure affect the boiling point Does Boiling Temperature Increase With Pressure As temperature increases, more particles have enough energy to escape to the gas phase. When a gas, the molecules occupy a far greater volume than they do when a liquid. For example, water will remain at 100ºc (at a pressure of 1 atm or. Therefore, increasing the pressure displaces the equilibrium (c.f. The boiling point of something is the point. Does Boiling Temperature Increase With Pressure.
From mavink.com
Water Boiling Point Pressure Chart Does Boiling Temperature Increase With Pressure As temperature increases, more particles have enough energy to escape to the gas phase. The boiling point of water depends. Therefore, increasing the pressure displaces the equilibrium (c.f. The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to atmospheric pressure. This increases the vapour pressure. For example, water will remain at 100ºc. Does Boiling Temperature Increase With Pressure.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Does Boiling Temperature Increase With Pressure The boiling point of water depends. The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to atmospheric pressure. The boiling point of something is the point where the vapour pressure equals or exceeds the atmospheric pressure, as stated. When the vapour pressure equals atmospheric. This increases the vapour pressure. As temperature increases,. Does Boiling Temperature Increase With Pressure.
From mavink.com
Water Boiling Pressure Chart Does Boiling Temperature Increase With Pressure As temperature increases, more particles have enough energy to escape to the gas phase. Therefore the temperature of the liquid remains constant during boiling. Therefore, increasing the pressure displaces the equilibrium (c.f. The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to atmospheric pressure. This increases the vapour pressure. 95 rows water. Does Boiling Temperature Increase With Pressure.
From sciencenotes.org
How to Boil Water at Room Temperature Does Boiling Temperature Increase With Pressure The boiling point of something is the point where the vapour pressure equals or exceeds the atmospheric pressure, as stated. This increases the vapour pressure. The boiling point of water depends. As temperature increases, more particles have enough energy to escape to the gas phase. 95 rows water boiling point in pressure higher than atm. When a gas, the molecules. Does Boiling Temperature Increase With Pressure.
From socratic.org
How do mountains affect temperature on both the windward side and the Does Boiling Temperature Increase With Pressure The boiling point of water depends. As temperature increases, more particles have enough energy to escape to the gas phase. The boiling point of something is the point where the vapour pressure equals or exceeds the atmospheric pressure, as stated. For example, water will remain at 100ºc (at a pressure of 1 atm or. 95 rows water boiling point in. Does Boiling Temperature Increase With Pressure.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Does Boiling Temperature Increase With Pressure The boiling point of water depends. 95 rows water boiling point in pressure higher than atm. As temperature increases, more particles have enough energy to escape to the gas phase. The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to atmospheric pressure. When a gas, the molecules occupy a far greater volume. Does Boiling Temperature Increase With Pressure.
From labbyag.es
Water Boiling Temperature Pressure Chart Labb by AG Does Boiling Temperature Increase With Pressure 95 rows water boiling point in pressure higher than atm. The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to atmospheric pressure. Therefore, increasing the pressure displaces the equilibrium (c.f. Therefore the temperature of the liquid remains constant during boiling. For example, water will remain at 100ºc (at a pressure of 1. Does Boiling Temperature Increase With Pressure.
From medium.com
Does water freeze or boil in space? Starts With A Bang! Medium Does Boiling Temperature Increase With Pressure This increases the vapour pressure. When a gas, the molecules occupy a far greater volume than they do when a liquid. 95 rows water boiling point in pressure higher than atm. Therefore the temperature of the liquid remains constant during boiling. Therefore, increasing the pressure displaces the equilibrium (c.f. When the vapour pressure equals atmospheric. The boiling point of something. Does Boiling Temperature Increase With Pressure.
From sciencenotes.org
Does Boiling Water Keep Getting Hotter? Does Boiling Temperature Increase With Pressure As temperature increases, more particles have enough energy to escape to the gas phase. This increases the vapour pressure. The boiling point of water depends. When a gas, the molecules occupy a far greater volume than they do when a liquid. The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to atmospheric. Does Boiling Temperature Increase With Pressure.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Does Boiling Temperature Increase With Pressure The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to atmospheric pressure. The boiling point of water at atmospheric pressure (101315 pa) is 100c, however the boiling point is changing with. For example, water will remain at 100ºc (at a pressure of 1 atm or. The boiling point of something is the. Does Boiling Temperature Increase With Pressure.
From www.researchgate.net
The vapor pressure of ethanol vs. the normal boilingpoint temperature Does Boiling Temperature Increase With Pressure The boiling point of water at atmospheric pressure (101315 pa) is 100c, however the boiling point is changing with. When a gas, the molecules occupy a far greater volume than they do when a liquid. The boiling point of something is the point where the vapour pressure equals or exceeds the atmospheric pressure, as stated. The normal boiling point is. Does Boiling Temperature Increase With Pressure.
From bionic-pirate.blogspot.com
Boiling Point Elouise Denny Does Boiling Temperature Increase With Pressure For example, water will remain at 100ºc (at a pressure of 1 atm or. As temperature increases, more particles have enough energy to escape to the gas phase. 95 rows water boiling point in pressure higher than atm. When a gas, the molecules occupy a far greater volume than they do when a liquid. The boiling point of something is. Does Boiling Temperature Increase With Pressure.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation Does Boiling Temperature Increase With Pressure The boiling point of water depends. When a gas, the molecules occupy a far greater volume than they do when a liquid. The boiling point of water at atmospheric pressure (101315 pa) is 100c, however the boiling point is changing with. 95 rows water boiling point in pressure higher than atm. When the vapour pressure equals atmospheric. The boiling point. Does Boiling Temperature Increase With Pressure.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Does Boiling Temperature Increase With Pressure The boiling point of water at atmospheric pressure (101315 pa) is 100c, however the boiling point is changing with. This increases the vapour pressure. When the vapour pressure equals atmospheric. The boiling point of something is the point where the vapour pressure equals or exceeds the atmospheric pressure, as stated. For example, water will remain at 100ºc (at a pressure. Does Boiling Temperature Increase With Pressure.
From cosmeticagoutier.com
Kokpunkt för vatten vilken temperatur kokar vatten? Blogger Value Does Boiling Temperature Increase With Pressure As temperature increases, more particles have enough energy to escape to the gas phase. Therefore the temperature of the liquid remains constant during boiling. The boiling point of water at atmospheric pressure (101315 pa) is 100c, however the boiling point is changing with. Therefore, increasing the pressure displaces the equilibrium (c.f. The normal boiling point is the temperature in which. Does Boiling Temperature Increase With Pressure.
From www.youtube.com
Boiling point and external pressure YouTube Does Boiling Temperature Increase With Pressure Therefore the temperature of the liquid remains constant during boiling. This increases the vapour pressure. The boiling point of water depends. 95 rows water boiling point in pressure higher than atm. When the vapour pressure equals atmospheric. The boiling point of something is the point where the vapour pressure equals or exceeds the atmospheric pressure, as stated. Therefore, increasing the. Does Boiling Temperature Increase With Pressure.