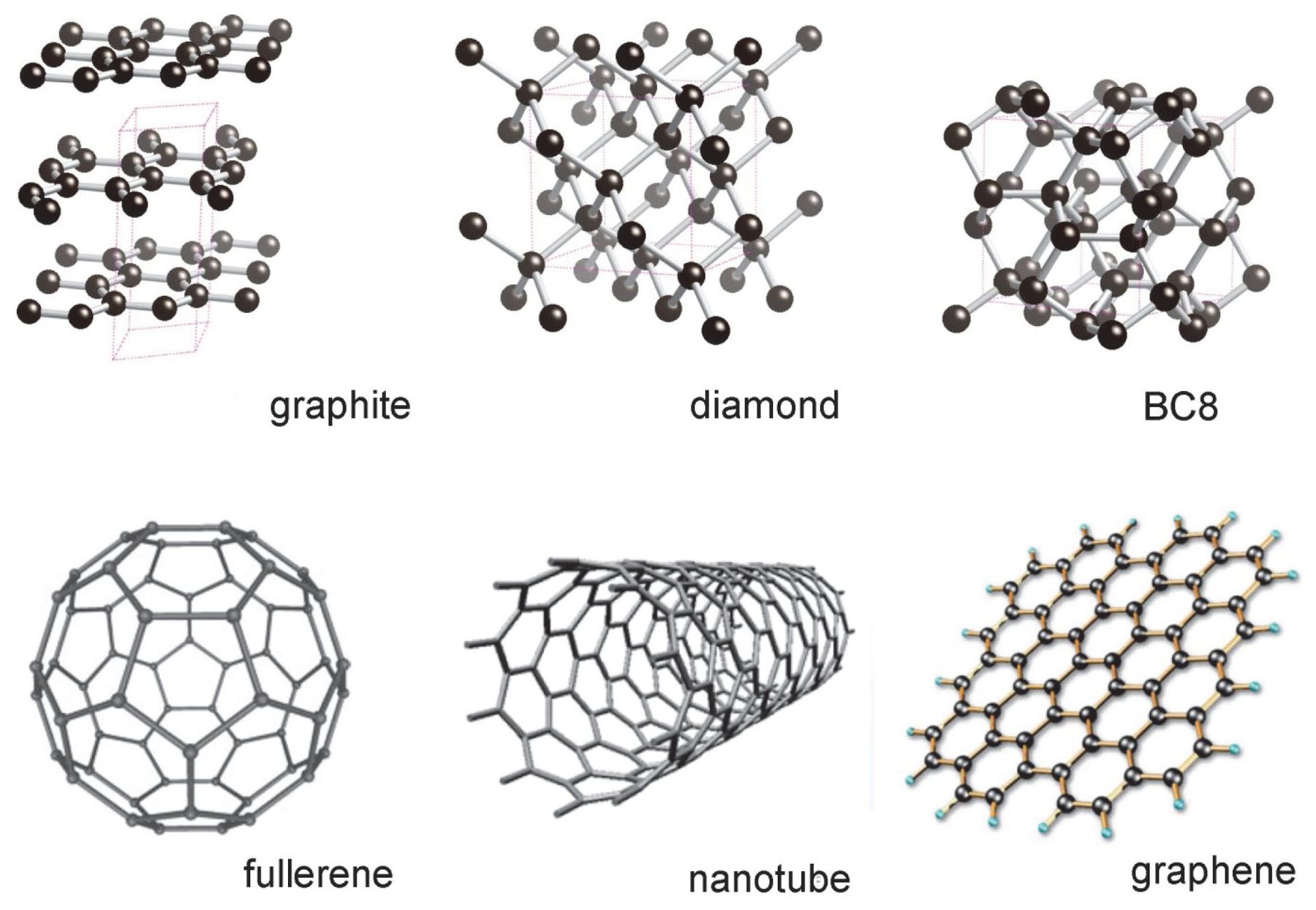
Graphite And Diamond . Learn about the structure, properties and uses of diamond and graphite, two different forms of carbon with covalent bonds. Although there are many differences between these two substances, the main difference between diamond and graphite is that diamond is made out of sp3 hybridized carbon atoms whereas graphite is made out of sp2 hybridized carbon atoms. Learn how diamond and graphite are different forms of carbon with distinct properties and structures. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon (iv) oxide). Compare and contrast their chemical and physical characteristics, such as hardness, conductivity, melting point and density. Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. What is the difference between diamond and graphite. Graphite and diamond are some of the most popular allotropes of carbon. Diamond and graphite are two allotropes of carbon: It is soft and slippery, and its hardness is less than one on the mohs. In today’s industrial applications, these two carbon. This page relates the structures of covalent network solids to the. Learn how diamond and graphite, both made of carbon, differ in crystal structure, hardness, thermal conductivity, and more. Pure forms of the same element that differ in structure. The system of carbon allotropes spans an astounding range of extremes, considering.
from
Find out how diamond is harder, more stable, and more valuable than graphite, and how they. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon (iv) oxide). What is the difference between diamond and graphite. Learn how diamond and graphite, both made of carbon, differ in crystal structure, hardness, thermal conductivity, and more. This page relates the structures of covalent network solids to the. The system of carbon allotropes spans an astounding range of extremes, considering. It is soft and slippery, and its hardness is less than one on the mohs. Learn how diamond and graphite are different forms of carbon with distinct properties and structures. Graphite and diamond are some of the most popular allotropes of carbon. Compare and contrast their chemical and physical characteristics, such as hardness, conductivity, melting point and density.
Graphite And Diamond Diamond and graphite are two allotropes of carbon: What is the difference between diamond and graphite. This page relates the structures of covalent network solids to the. It is soft and slippery, and its hardness is less than one on the mohs. Learn how diamond and graphite are different forms of carbon with distinct properties and structures. In today’s industrial applications, these two carbon. Learn how diamond and graphite, both made of carbon, differ in crystal structure, hardness, thermal conductivity, and more. Although there are many differences between these two substances, the main difference between diamond and graphite is that diamond is made out of sp3 hybridized carbon atoms whereas graphite is made out of sp2 hybridized carbon atoms. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon (iv) oxide). Pure forms of the same element that differ in structure. Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. Diamond and graphite are two allotropes of carbon: Learn about the structure, properties and uses of diamond and graphite, two different forms of carbon with covalent bonds. Compare and contrast their chemical and physical characteristics, such as hardness, conductivity, melting point and density. Find out how diamond is harder, more stable, and more valuable than graphite, and how they. The system of carbon allotropes spans an astounding range of extremes, considering.
From www.petragems.com
Difference Between Diamond and Graphite Graphite And Diamond What is the difference between diamond and graphite. In today’s industrial applications, these two carbon. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon (iv) oxide). Compare and contrast their chemical and physical characteristics, such as hardness, conductivity, melting point and density. Pure forms of the same element that differ in structure. This page relates. Graphite And Diamond.
From
Graphite And Diamond The system of carbon allotropes spans an astounding range of extremes, considering. Pure forms of the same element that differ in structure. Find out how diamond is harder, more stable, and more valuable than graphite, and how they. Graphite and diamond are some of the most popular allotropes of carbon. Diamond and graphite are two allotropes of carbon: This page. Graphite And Diamond.
From fphoto.photoshelter.com
kimberlite diamond igneous rock graphite comparison Fundamental Graphite And Diamond Pure forms of the same element that differ in structure. Find out how diamond is harder, more stable, and more valuable than graphite, and how they. It is soft and slippery, and its hardness is less than one on the mohs. Compare and contrast their chemical and physical characteristics, such as hardness, conductivity, melting point and density. Learn how diamond. Graphite And Diamond.
From
Graphite And Diamond Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon (iv) oxide). In today’s industrial applications, these two carbon. Although there are many differences between these two substances, the main difference between diamond and graphite is that diamond is made out of sp3 hybridized carbon atoms whereas graphite is made out of sp2 hybridized carbon atoms.. Graphite And Diamond.
From
Graphite And Diamond This page relates the structures of covalent network solids to the. Diamond and graphite are two allotropes of carbon: The system of carbon allotropes spans an astounding range of extremes, considering. It is soft and slippery, and its hardness is less than one on the mohs. Learn how diamond and graphite are different forms of carbon with distinct properties and. Graphite And Diamond.
From ar.inspiredpencil.com
Diamond Mineral Graphite And Diamond Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. It is soft and slippery, and its hardness is less than one on the mohs. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon (iv) oxide). The system of carbon allotropes spans an astounding range of extremes, considering.. Graphite And Diamond.
From
Graphite And Diamond The system of carbon allotropes spans an astounding range of extremes, considering. Graphite and diamond are some of the most popular allotropes of carbon. Learn about the structure, properties and uses of diamond and graphite, two different forms of carbon with covalent bonds. This page relates the structures of covalent network solids to the. Diamond and graphite are two allotropes. Graphite And Diamond.
From
Graphite And Diamond Learn about the structure, properties and uses of diamond and graphite, two different forms of carbon with covalent bonds. Diamond and graphite are two allotropes of carbon: It is soft and slippery, and its hardness is less than one on the mohs. Compare and contrast their chemical and physical characteristics, such as hardness, conductivity, melting point and density. Although there. Graphite And Diamond.
From zh.wikipedia.org
FileDiamond and graphite.jpg 维基百科,自由的百科全书 Graphite And Diamond It is soft and slippery, and its hardness is less than one on the mohs. Pure forms of the same element that differ in structure. Diamond and graphite are two allotropes of carbon: Learn how diamond and graphite are different forms of carbon with distinct properties and structures. The system of carbon allotropes spans an astounding range of extremes, considering.. Graphite And Diamond.
From
Graphite And Diamond This page relates the structures of covalent network solids to the. Find out how diamond is harder, more stable, and more valuable than graphite, and how they. Pure forms of the same element that differ in structure. Learn how diamond and graphite are different forms of carbon with distinct properties and structures. Compare and contrast their chemical and physical characteristics,. Graphite And Diamond.
From animalia-life.club
Graphite Coal Diamond Graphite And Diamond Graphite and diamond are some of the most popular allotropes of carbon. Although there are many differences between these two substances, the main difference between diamond and graphite is that diamond is made out of sp3 hybridized carbon atoms whereas graphite is made out of sp2 hybridized carbon atoms. Learn how diamond and graphite are different forms of carbon with. Graphite And Diamond.
From ar.inspiredpencil.com
Diamond And Graphite Structure Graphite And Diamond Find out how diamond is harder, more stable, and more valuable than graphite, and how they. Learn how diamond and graphite, both made of carbon, differ in crystal structure, hardness, thermal conductivity, and more. Although there are many differences between these two substances, the main difference between diamond and graphite is that diamond is made out of sp3 hybridized carbon. Graphite And Diamond.
From
Graphite And Diamond It is soft and slippery, and its hardness is less than one on the mohs. Graphite and diamond are some of the most popular allotropes of carbon. Compare and contrast their chemical and physical characteristics, such as hardness, conductivity, melting point and density. Learn about the structure, properties and uses of diamond and graphite, two different forms of carbon with. Graphite And Diamond.
From
Graphite And Diamond Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. Graphite and diamond are some of the most popular allotropes of carbon. Find out how diamond is harder, more stable, and more valuable than graphite, and how they. Although there are many differences between these two substances, the main difference between diamond and. Graphite And Diamond.
From www.pinterest.jp
Scientists Turned Diamond Into Graphite for the First Time Gem Diamonds Graphite And Diamond Learn how diamond and graphite are different forms of carbon with distinct properties and structures. Compare and contrast their chemical and physical characteristics, such as hardness, conductivity, melting point and density. Although there are many differences between these two substances, the main difference between diamond and graphite is that diamond is made out of sp3 hybridized carbon atoms whereas graphite. Graphite And Diamond.
From
Graphite And Diamond The system of carbon allotropes spans an astounding range of extremes, considering. This page relates the structures of covalent network solids to the. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon (iv) oxide). Compare and contrast their chemical and physical characteristics, such as hardness, conductivity, melting point and density. Learn about the structure, properties. Graphite And Diamond.
From ar.inspiredpencil.com
Diamond And Graphite Structure Graphite And Diamond Compare and contrast their chemical and physical characteristics, such as hardness, conductivity, melting point and density. This page relates the structures of covalent network solids to the. Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. Learn how diamond and graphite, both made of carbon, differ in crystal structure, hardness, thermal conductivity,. Graphite And Diamond.
From
Graphite And Diamond What is the difference between diamond and graphite. It is soft and slippery, and its hardness is less than one on the mohs. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon (iv) oxide). Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. Graphite and diamond are. Graphite And Diamond.
From www.youtube.com
Diamond vs Graphite Quick differences and comparison YouTube Graphite And Diamond Graphite and diamond are some of the most popular allotropes of carbon. Find out how diamond is harder, more stable, and more valuable than graphite, and how they. Diamond and graphite are two allotropes of carbon: In today’s industrial applications, these two carbon. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon (iv) oxide). Pure. Graphite And Diamond.
From
Graphite And Diamond Learn how diamond and graphite, both made of carbon, differ in crystal structure, hardness, thermal conductivity, and more. Pure forms of the same element that differ in structure. It is soft and slippery, and its hardness is less than one on the mohs. Learn how diamond and graphite are different forms of carbon with distinct properties and structures. Covalent network. Graphite And Diamond.
From
Graphite And Diamond The system of carbon allotropes spans an astounding range of extremes, considering. Learn how diamond and graphite are different forms of carbon with distinct properties and structures. Find out how diamond is harder, more stable, and more valuable than graphite, and how they. Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily.. Graphite And Diamond.
From www.alamy.com
Carbon graphite and diamond hires stock photography and images Alamy Graphite And Diamond Diamond and graphite are two allotropes of carbon: Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. Learn how diamond and graphite are different forms of carbon with distinct properties and structures. Learn about the structure, properties and uses of diamond and graphite, two different forms of carbon with covalent bonds. It. Graphite And Diamond.
From
Graphite And Diamond Diamond and graphite are two allotropes of carbon: In today’s industrial applications, these two carbon. Pure forms of the same element that differ in structure. The system of carbon allotropes spans an astounding range of extremes, considering. Learn about the structure, properties and uses of diamond and graphite, two different forms of carbon with covalent bonds. Learn how diamond and. Graphite And Diamond.
From ar.inspiredpencil.com
Diamond And Graphite Structure Graphite And Diamond It is soft and slippery, and its hardness is less than one on the mohs. Find out how diamond is harder, more stable, and more valuable than graphite, and how they. Pure forms of the same element that differ in structure. What is the difference between diamond and graphite. Unlike diamond, graphite can be used as a lubricant or in. Graphite And Diamond.
From
Graphite And Diamond Graphite and diamond are some of the most popular allotropes of carbon. Although there are many differences between these two substances, the main difference between diamond and graphite is that diamond is made out of sp3 hybridized carbon atoms whereas graphite is made out of sp2 hybridized carbon atoms. Diamond and graphite are two allotropes of carbon: Learn how diamond. Graphite And Diamond.
From ar.inspiredpencil.com
Carbon Structure Of Diamond And Graphite Graphite And Diamond Graphite and diamond are some of the most popular allotropes of carbon. Learn how diamond and graphite, both made of carbon, differ in crystal structure, hardness, thermal conductivity, and more. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon (iv) oxide). This page relates the structures of covalent network solids to the. The system of. Graphite And Diamond.
From
Graphite And Diamond This page relates the structures of covalent network solids to the. Compare and contrast their chemical and physical characteristics, such as hardness, conductivity, melting point and density. Graphite and diamond are some of the most popular allotropes of carbon. Pure forms of the same element that differ in structure. Learn about the structure, properties and uses of diamond and graphite,. Graphite And Diamond.
From animalia-life.club
Graphite Coal Diamond Graphite And Diamond Find out how diamond is harder, more stable, and more valuable than graphite, and how they. Learn how diamond and graphite are different forms of carbon with distinct properties and structures. Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. The system of carbon allotropes spans an astounding range of extremes, considering.. Graphite And Diamond.
From
Graphite And Diamond Learn how diamond and graphite, both made of carbon, differ in crystal structure, hardness, thermal conductivity, and more. Graphite and diamond are some of the most popular allotropes of carbon. The system of carbon allotropes spans an astounding range of extremes, considering. Find out how diamond is harder, more stable, and more valuable than graphite, and how they. Diamond and. Graphite And Diamond.
From
Graphite And Diamond Although there are many differences between these two substances, the main difference between diamond and graphite is that diamond is made out of sp3 hybridized carbon atoms whereas graphite is made out of sp2 hybridized carbon atoms. Learn how diamond and graphite, both made of carbon, differ in crystal structure, hardness, thermal conductivity, and more. What is the difference between. Graphite And Diamond.
From
Graphite And Diamond The system of carbon allotropes spans an astounding range of extremes, considering. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon (iv) oxide). Although there are many differences between these two substances, the main difference between diamond and graphite is that diamond is made out of sp3 hybridized carbon atoms whereas graphite is made out. Graphite And Diamond.
From
Graphite And Diamond Learn how diamond and graphite, both made of carbon, differ in crystal structure, hardness, thermal conductivity, and more. It is soft and slippery, and its hardness is less than one on the mohs. In today’s industrial applications, these two carbon. Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. Although there are. Graphite And Diamond.
From fphoto.photoshelter.com
science chemistry carbon allotrope graphite diamond Fundamental Graphite And Diamond Find out how diamond is harder, more stable, and more valuable than graphite, and how they. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon (iv) oxide). It is soft and slippery, and its hardness is less than one on the mohs. The system of carbon allotropes spans an astounding range of extremes, considering. Learn. Graphite And Diamond.
From www.slideshare.net
Diamond & Graphite Graphite And Diamond What is the difference between diamond and graphite. Find out how diamond is harder, more stable, and more valuable than graphite, and how they. In today’s industrial applications, these two carbon. Diamond and graphite are two allotropes of carbon: It is soft and slippery, and its hardness is less than one on the mohs. Learn how diamond and graphite, both. Graphite And Diamond.
From
Graphite And Diamond What is the difference between diamond and graphite. In today’s industrial applications, these two carbon. Learn about the structure, properties and uses of diamond and graphite, two different forms of carbon with covalent bonds. The system of carbon allotropes spans an astounding range of extremes, considering. Pure forms of the same element that differ in structure. Unlike diamond, graphite can. Graphite And Diamond.