How To Tell If A Solution Is An Electrolyte . Learn how to identify electrolytes based on their name or structure and how they dissociate in water. When trying to determine if a substance is an electrolyte vs. A compound that conducts an electric current when it is in an aqueous solution or melted. The conductivity of an electrolyte solution is related to the strength of the electrolyte. In a solution, \ (\ce {h2co3}\) molecules are present. Find examples of strong, weak, and non electrolytes and their. Solutions of electrolytes contain ions that permit the passage of electricity. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrolytic solutions are those that are capable of conducting an electric current. Does it conduct electricity when melted or dissolved? Nonelectrolyte, the question that must be answered is: A substance that, when added to water, renders it conductive, is. The fraction (often expressed as a %) that undergos ionization depends on the concentration of the solution.
from www.slideserve.com
A compound that conducts an electric current when it is in an aqueous solution or melted. Find examples of strong, weak, and non electrolytes and their. Does it conduct electricity when melted or dissolved? Electrolytic solutions are those that are capable of conducting an electric current. Solutions of electrolytes contain ions that permit the passage of electricity. In a solution, \ (\ce {h2co3}\) molecules are present. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Nonelectrolyte, the question that must be answered is: The fraction (often expressed as a %) that undergos ionization depends on the concentration of the solution. When trying to determine if a substance is an electrolyte vs.
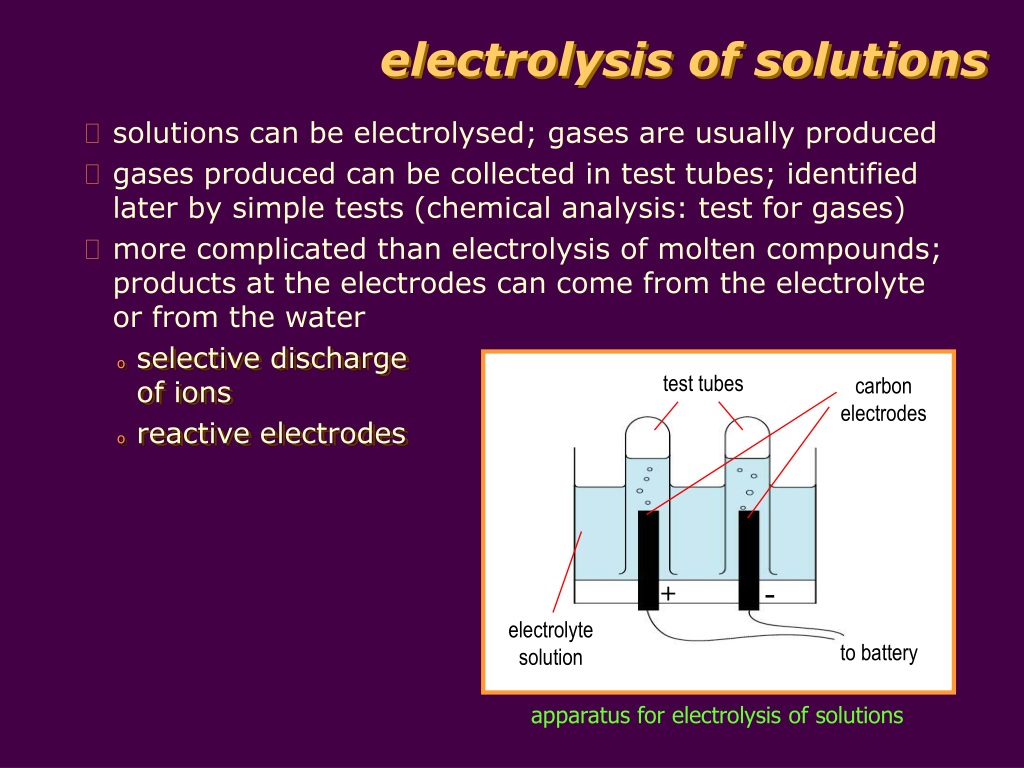
PPT electrolysis of solutions PowerPoint Presentation, free download
How To Tell If A Solution Is An Electrolyte Learn how to identify electrolytes based on their name or structure and how they dissociate in water. The conductivity of an electrolyte solution is related to the strength of the electrolyte. Nonelectrolyte, the question that must be answered is: When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Does it conduct electricity when melted or dissolved? Find examples of strong, weak, and non electrolytes and their. Learn how to identify electrolytes based on their name or structure and how they dissociate in water. In a solution, \ (\ce {h2co3}\) molecules are present. Electrolytic solutions are those that are capable of conducting an electric current. The fraction (often expressed as a %) that undergos ionization depends on the concentration of the solution. Solutions of electrolytes contain ions that permit the passage of electricity. A compound that conducts an electric current when it is in an aqueous solution or melted. When trying to determine if a substance is an electrolyte vs. A substance that, when added to water, renders it conductive, is.
From general.chemistrysteps.com
General Properties of Solutions Chemistry Steps How To Tell If A Solution Is An Electrolyte A compound that conducts an electric current when it is in an aqueous solution or melted. In a solution, \ (\ce {h2co3}\) molecules are present. Electrolytic solutions are those that are capable of conducting an electric current. A substance that, when added to water, renders it conductive, is. Find examples of strong, weak, and non electrolytes and their. Learn how. How To Tell If A Solution Is An Electrolyte.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors How To Tell If A Solution Is An Electrolyte The conductivity of an electrolyte solution is related to the strength of the electrolyte. Electrolytic solutions are those that are capable of conducting an electric current. Does it conduct electricity when melted or dissolved? Nonelectrolyte, the question that must be answered is: Find examples of strong, weak, and non electrolytes and their. When some substances are dissolved in water, they. How To Tell If A Solution Is An Electrolyte.
From www.pathwaystochemistry.com
Electrolytes and Nonelectrolytes Pathways to Chemistry How To Tell If A Solution Is An Electrolyte Learn how to identify electrolytes based on their name or structure and how they dissociate in water. A compound that conducts an electric current when it is in an aqueous solution or melted. Find examples of strong, weak, and non electrolytes and their. Electrolytic solutions are those that are capable of conducting an electric current. In a solution, \ (\ce. How To Tell If A Solution Is An Electrolyte.
From desmondgrodelgado.blogspot.com
How Can You Tell if a Compound Is an Electrolyte DesmondgroDelgado How To Tell If A Solution Is An Electrolyte In a solution, \ (\ce {h2co3}\) molecules are present. Learn how to identify electrolytes based on their name or structure and how they dissociate in water. Electrolytic solutions are those that are capable of conducting an electric current. When trying to determine if a substance is an electrolyte vs. Does it conduct electricity when melted or dissolved? The conductivity of. How To Tell If A Solution Is An Electrolyte.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types How To Tell If A Solution Is An Electrolyte When trying to determine if a substance is an electrolyte vs. Electrolytic solutions are those that are capable of conducting an electric current. The fraction (often expressed as a %) that undergos ionization depends on the concentration of the solution. A substance that, when added to water, renders it conductive, is. When some substances are dissolved in water, they undergo. How To Tell If A Solution Is An Electrolyte.
From slideplayer.com
Solutes Electrolytes and Nonelectrolytes ppt download How To Tell If A Solution Is An Electrolyte Learn how to identify electrolytes based on their name or structure and how they dissociate in water. When trying to determine if a substance is an electrolyte vs. A compound that conducts an electric current when it is in an aqueous solution or melted. The fraction (often expressed as a %) that undergos ionization depends on the concentration of the. How To Tell If A Solution Is An Electrolyte.
From in.pinterest.com
Pin on Acids Bases and Solutions How To Tell If A Solution Is An Electrolyte A substance that, when added to water, renders it conductive, is. Learn how to identify electrolytes based on their name or structure and how they dissociate in water. The fraction (often expressed as a %) that undergos ionization depends on the concentration of the solution. Solutions of electrolytes contain ions that permit the passage of electricity. A compound that conducts. How To Tell If A Solution Is An Electrolyte.
From webmis.highland.cc.il.us
Electrolysis How To Tell If A Solution Is An Electrolyte Electrolytic solutions are those that are capable of conducting an electric current. A compound that conducts an electric current when it is in an aqueous solution or melted. Find examples of strong, weak, and non electrolytes and their. Nonelectrolyte, the question that must be answered is: The fraction (often expressed as a %) that undergos ionization depends on the concentration. How To Tell If A Solution Is An Electrolyte.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download How To Tell If A Solution Is An Electrolyte When trying to determine if a substance is an electrolyte vs. In a solution, \ (\ce {h2co3}\) molecules are present. Find examples of strong, weak, and non electrolytes and their. Solutions of electrolytes contain ions that permit the passage of electricity. Does it conduct electricity when melted or dissolved? Nonelectrolyte, the question that must be answered is: The fraction (often. How To Tell If A Solution Is An Electrolyte.
From www.slideserve.com
PPT Chapter 4 Aqueous Reactions and Solution Stoichiometry How To Tell If A Solution Is An Electrolyte The fraction (often expressed as a %) that undergos ionization depends on the concentration of the solution. Solutions of electrolytes contain ions that permit the passage of electricity. Does it conduct electricity when melted or dissolved? The conductivity of an electrolyte solution is related to the strength of the electrolyte. Find examples of strong, weak, and non electrolytes and their.. How To Tell If A Solution Is An Electrolyte.
From webmis.highland.cc.il.us
Aqueous Solutions How To Tell If A Solution Is An Electrolyte When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Learn how to identify electrolytes based on their name or structure and how they dissociate in water. Electrolytic solutions are those that are capable of conducting an electric current. A substance that, when added to water, renders it conductive,. How To Tell If A Solution Is An Electrolyte.
From study.com
Electrolysis of Aqueous Solutions Lesson How To Tell If A Solution Is An Electrolyte The conductivity of an electrolyte solution is related to the strength of the electrolyte. The fraction (often expressed as a %) that undergos ionization depends on the concentration of the solution. In a solution, \ (\ce {h2co3}\) molecules are present. Electrolytic solutions are those that are capable of conducting an electric current. Find examples of strong, weak, and non electrolytes. How To Tell If A Solution Is An Electrolyte.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes How To Tell If A Solution Is An Electrolyte Find examples of strong, weak, and non electrolytes and their. A substance that, when added to water, renders it conductive, is. When trying to determine if a substance is an electrolyte vs. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Nonelectrolyte, the question that must be answered. How To Tell If A Solution Is An Electrolyte.
From ar.inspiredpencil.com
Electrolyte Examples Chemistry How To Tell If A Solution Is An Electrolyte When trying to determine if a substance is an electrolyte vs. A substance that, when added to water, renders it conductive, is. Find examples of strong, weak, and non electrolytes and their. Electrolytic solutions are those that are capable of conducting an electric current. A compound that conducts an electric current when it is in an aqueous solution or melted.. How To Tell If A Solution Is An Electrolyte.
From www.nagwa.com
Question Video Identifying What Type of Electrolyte Sodium Chloride Is How To Tell If A Solution Is An Electrolyte Learn how to identify electrolytes based on their name or structure and how they dissociate in water. The conductivity of an electrolyte solution is related to the strength of the electrolyte. The fraction (often expressed as a %) that undergos ionization depends on the concentration of the solution. Nonelectrolyte, the question that must be answered is: Does it conduct electricity. How To Tell If A Solution Is An Electrolyte.
From www.pinterest.com
Electrolyte Easy Science Electrolytes, Easy science, Flashcards How To Tell If A Solution Is An Electrolyte Does it conduct electricity when melted or dissolved? Nonelectrolyte, the question that must be answered is: A substance that, when added to water, renders it conductive, is. The conductivity of an electrolyte solution is related to the strength of the electrolyte. Electrolytic solutions are those that are capable of conducting an electric current. When trying to determine if a substance. How To Tell If A Solution Is An Electrolyte.
From socratic.org
Question a0b96 + Example How To Tell If A Solution Is An Electrolyte The fraction (often expressed as a %) that undergos ionization depends on the concentration of the solution. In a solution, \ (\ce {h2co3}\) molecules are present. When trying to determine if a substance is an electrolyte vs. Nonelectrolyte, the question that must be answered is: When some substances are dissolved in water, they undergo either a physical or a chemical. How To Tell If A Solution Is An Electrolyte.
From www.shutterstock.com
413 Electrolytic solution Gambar, Foto Stok & Vektor Shutterstock How To Tell If A Solution Is An Electrolyte Electrolytic solutions are those that are capable of conducting an electric current. A compound that conducts an electric current when it is in an aqueous solution or melted. The conductivity of an electrolyte solution is related to the strength of the electrolyte. A substance that, when added to water, renders it conductive, is. Solutions of electrolytes contain ions that permit. How To Tell If A Solution Is An Electrolyte.
From ar.inspiredpencil.com
Electrolyte Examples Chemistry How To Tell If A Solution Is An Electrolyte Learn how to identify electrolytes based on their name or structure and how they dissociate in water. Nonelectrolyte, the question that must be answered is: In a solution, \ (\ce {h2co3}\) molecules are present. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. When trying to determine if. How To Tell If A Solution Is An Electrolyte.
From www.slideserve.com
PPT Solutions Review Pt 2 PowerPoint Presentation, free download ID How To Tell If A Solution Is An Electrolyte Nonelectrolyte, the question that must be answered is: Electrolytic solutions are those that are capable of conducting an electric current. A compound that conducts an electric current when it is in an aqueous solution or melted. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Find examples of. How To Tell If A Solution Is An Electrolyte.
From www.slideserve.com
PPT Strong and Weak Electrolytes PowerPoint Presentation, free How To Tell If A Solution Is An Electrolyte A compound that conducts an electric current when it is in an aqueous solution or melted. When trying to determine if a substance is an electrolyte vs. The fraction (often expressed as a %) that undergos ionization depends on the concentration of the solution. Learn how to identify electrolytes based on their name or structure and how they dissociate in. How To Tell If A Solution Is An Electrolyte.
From www.slideserve.com
PPT Aqueoussolution Reactions PowerPoint Presentation ID159152 How To Tell If A Solution Is An Electrolyte In a solution, \ (\ce {h2co3}\) molecules are present. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. A compound that conducts an electric current when it is in an aqueous solution or melted. When trying to determine if a substance is an electrolyte vs. The conductivity of. How To Tell If A Solution Is An Electrolyte.
From www.ck12.org
Solvation, Dissociation, and Electrolytes Example 4 ( Video How To Tell If A Solution Is An Electrolyte Solutions of electrolytes contain ions that permit the passage of electricity. Electrolytic solutions are those that are capable of conducting an electric current. Find examples of strong, weak, and non electrolytes and their. The conductivity of an electrolyte solution is related to the strength of the electrolyte. Does it conduct electricity when melted or dissolved? In a solution, \ (\ce. How To Tell If A Solution Is An Electrolyte.
From socratic.org
Question 0b448 Socratic How To Tell If A Solution Is An Electrolyte A compound that conducts an electric current when it is in an aqueous solution or melted. When trying to determine if a substance is an electrolyte vs. Find examples of strong, weak, and non electrolytes and their. Solutions of electrolytes contain ions that permit the passage of electricity. When some substances are dissolved in water, they undergo either a physical. How To Tell If A Solution Is An Electrolyte.
From www.britannica.com
Electric current in a solution of electrolytes studied Britannica How To Tell If A Solution Is An Electrolyte Learn how to identify electrolytes based on their name or structure and how they dissociate in water. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. A substance that, when added to water, renders it conductive, is. Find examples of strong, weak, and non electrolytes and their. Does. How To Tell If A Solution Is An Electrolyte.
From www.youtube.com
Identifying Strong Electrolytes, Weak Electrolytes, and Nonelectrolytes How To Tell If A Solution Is An Electrolyte Find examples of strong, weak, and non electrolytes and their. A substance that, when added to water, renders it conductive, is. Nonelectrolyte, the question that must be answered is: When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. The conductivity of an electrolyte solution is related to the. How To Tell If A Solution Is An Electrolyte.
From www.slideserve.com
PPT Electrolytes PowerPoint Presentation, free download ID181760 How To Tell If A Solution Is An Electrolyte The fraction (often expressed as a %) that undergos ionization depends on the concentration of the solution. Find examples of strong, weak, and non electrolytes and their. Electrolytic solutions are those that are capable of conducting an electric current. Solutions of electrolytes contain ions that permit the passage of electricity. A compound that conducts an electric current when it is. How To Tell If A Solution Is An Electrolyte.
From killianxinicholson.blogspot.com
Which of the Following Is an Electrolyte Solution How To Tell If A Solution Is An Electrolyte In a solution, \ (\ce {h2co3}\) molecules are present. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. A substance that, when added to water, renders it conductive, is. Solutions of electrolytes contain ions that permit the passage of electricity. When trying to determine if a substance is. How To Tell If A Solution Is An Electrolyte.
From quizunbegotten.z21.web.core.windows.net
How To Identify Electrolytes How To Tell If A Solution Is An Electrolyte When trying to determine if a substance is an electrolyte vs. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. The conductivity of an electrolyte solution is related to the strength of the electrolyte. The fraction (often expressed as a %) that undergos ionization depends on the concentration. How To Tell If A Solution Is An Electrolyte.
From ar.inspiredpencil.com
Electrolyte Examples Chemistry How To Tell If A Solution Is An Electrolyte A substance that, when added to water, renders it conductive, is. The conductivity of an electrolyte solution is related to the strength of the electrolyte. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. When trying to determine if a substance is an electrolyte vs. Learn how to. How To Tell If A Solution Is An Electrolyte.
From www.shutterstock.com
19 Strong Electrolyte And Weak Electrolyte Images, Stock Photos How To Tell If A Solution Is An Electrolyte The conductivity of an electrolyte solution is related to the strength of the electrolyte. When trying to determine if a substance is an electrolyte vs. Learn how to identify electrolytes based on their name or structure and how they dissociate in water. Nonelectrolyte, the question that must be answered is: Electrolytic solutions are those that are capable of conducting an. How To Tell If A Solution Is An Electrolyte.
From www.batterypowertips.com
What is an electrolyte? Battery Power Tips How To Tell If A Solution Is An Electrolyte The fraction (often expressed as a %) that undergos ionization depends on the concentration of the solution. In a solution, \ (\ce {h2co3}\) molecules are present. A substance that, when added to water, renders it conductive, is. When trying to determine if a substance is an electrolyte vs. Electrolytic solutions are those that are capable of conducting an electric current.. How To Tell If A Solution Is An Electrolyte.
From www.numerade.com
SOLVED Consider the following diagrams for an aqueous solution of a How To Tell If A Solution Is An Electrolyte When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. When trying to determine if a substance is an electrolyte vs. A compound that conducts an electric current when it is in an aqueous solution or melted. A substance that, when added to water, renders it conductive, is. The. How To Tell If A Solution Is An Electrolyte.
From psiberg.com
Strong vs. Weak Electrolytes How to Categorize the Electrolytes? PSIBERG How To Tell If A Solution Is An Electrolyte The fraction (often expressed as a %) that undergos ionization depends on the concentration of the solution. Does it conduct electricity when melted or dissolved? Find examples of strong, weak, and non electrolytes and their. The conductivity of an electrolyte solution is related to the strength of the electrolyte. When some substances are dissolved in water, they undergo either a. How To Tell If A Solution Is An Electrolyte.
From hayden-relbrewer.blogspot.com
HaydenRelBrewer How To Tell If A Solution Is An Electrolyte Solutions of electrolytes contain ions that permit the passage of electricity. The conductivity of an electrolyte solution is related to the strength of the electrolyte. Electrolytic solutions are those that are capable of conducting an electric current. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. The fraction. How To Tell If A Solution Is An Electrolyte.