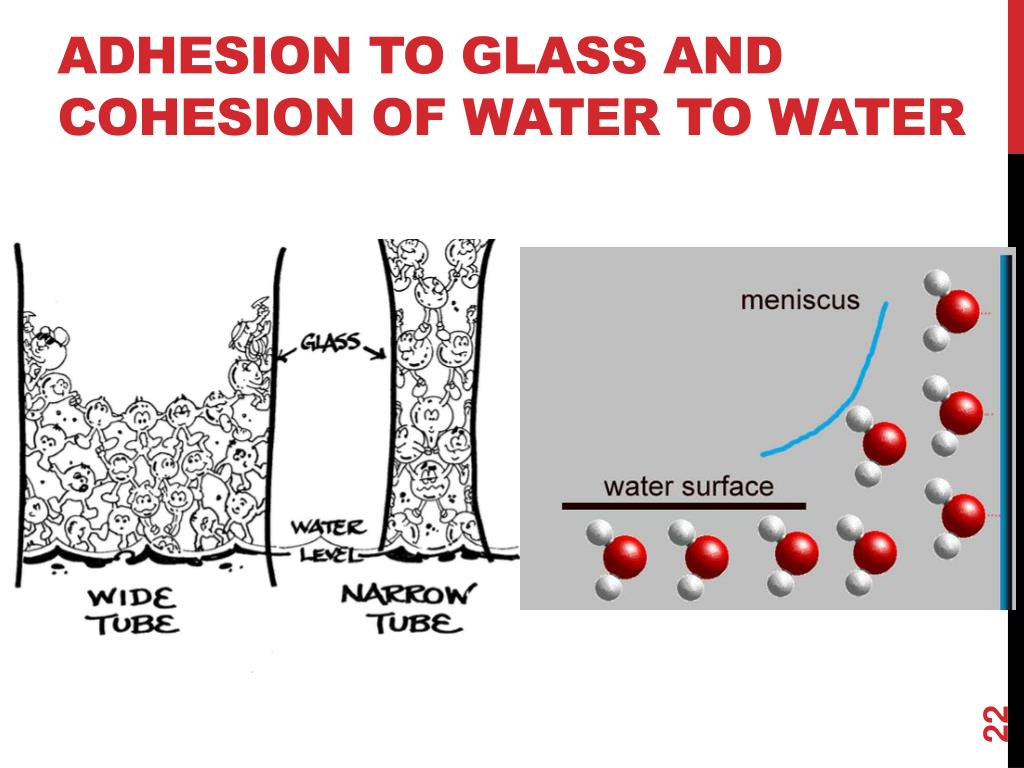
Why Is Water Cohesive And Adhesive . Provide examples of water’s cohesive and adhesive properties; Why are cohesive and adhesive forces important for life? One property is cohesion, the tendency for water molecules to stick together. Cohesion causes water to have surface tension, the property that. Discuss the role of acids, bases, and buffers in homeostasis Water molecules are very cohesive because of the molecule's polarity. Explain why water is an excellent solvent; However, when water is filled to the tip of the cylinder, the water level could maintain higher than the wall of the cylinder without pouring out resembling a concave down meniscus. Cohesion allows substances to withstand rupture when placed under stress while adhesion is the attraction between water and other molecules. Provide examples of water’s cohesive and adhesive properties; Discuss the role of acids, bases, and buffers in homeostasis Explain why water is an excellent solvent; Water is sticky and clumps together into drops because of its cohesive properties, but chemistry and. This is why you can fill a glass of water just barely above the rim without. Cohesive and adhesive forces are important for the transport of water from the.
from www.slideserve.com
Explain why water is an excellent solvent; Discuss the role of acids, bases, and buffers in homeostasis Water is sticky and clumps together into drops because of its cohesive properties, but chemistry and. Cohesive and adhesive forces are important for the transport of water from the. Discuss the role of acids, bases, and buffers in homeostasis Cohesion allows substances to withstand rupture when placed under stress while adhesion is the attraction between water and other molecules. Provide examples of water’s cohesive and adhesive properties; Explain why water is an excellent solvent; Provide examples of water’s cohesive and adhesive properties; Why are cohesive and adhesive forces important for life?
PPT Water and It’s properties PowerPoint Presentation, free download
Why Is Water Cohesive And Adhesive Water is sticky and clumps together into drops because of its cohesive properties, but chemistry and. This is why you can fill a glass of water just barely above the rim without. Provide examples of water’s cohesive and adhesive properties; Cohesive and adhesive forces are important for the transport of water from the. Discuss the role of acids, bases, and buffers in homeostasis However, when water is filled to the tip of the cylinder, the water level could maintain higher than the wall of the cylinder without pouring out resembling a concave down meniscus. Explain why water is an excellent solvent; Water molecules are very cohesive because of the molecule's polarity. Explain why water is an excellent solvent; Provide examples of water’s cohesive and adhesive properties; Why are cohesive and adhesive forces important for life? One property is cohesion, the tendency for water molecules to stick together. Cohesion causes water to have surface tension, the property that. Discuss the role of acids, bases, and buffers in homeostasis Cohesion allows substances to withstand rupture when placed under stress while adhesion is the attraction between water and other molecules. Water is sticky and clumps together into drops because of its cohesive properties, but chemistry and.
From healthsystem.netlify.app
Adhesion of water definition Why Is Water Cohesive And Adhesive This is why you can fill a glass of water just barely above the rim without. Discuss the role of acids, bases, and buffers in homeostasis Cohesion allows substances to withstand rupture when placed under stress while adhesion is the attraction between water and other molecules. Water molecules are very cohesive because of the molecule's polarity. However, when water is. Why Is Water Cohesive And Adhesive.
From www.slideshare.net
Properties of Water PowerPoint, Adhesion, Cohesion, Surface Tension Why Is Water Cohesive And Adhesive Provide examples of water’s cohesive and adhesive properties; This is why you can fill a glass of water just barely above the rim without. Water is sticky and clumps together into drops because of its cohesive properties, but chemistry and. Explain why water is an excellent solvent; Cohesive and adhesive forces are important for the transport of water from the.. Why Is Water Cohesive And Adhesive.
From ar.inspiredpencil.com
Adhesion Of Water Diagram Why Is Water Cohesive And Adhesive Why are cohesive and adhesive forces important for life? One property is cohesion, the tendency for water molecules to stick together. Water molecules are very cohesive because of the molecule's polarity. Water is sticky and clumps together into drops because of its cohesive properties, but chemistry and. Explain why water is an excellent solvent; Discuss the role of acids, bases,. Why Is Water Cohesive And Adhesive.
From www.pearson.com
Properties of Water Cohesion and Adhesion Pearson+ Channels Why Is Water Cohesive And Adhesive Why are cohesive and adhesive forces important for life? However, when water is filled to the tip of the cylinder, the water level could maintain higher than the wall of the cylinder without pouring out resembling a concave down meniscus. Water is sticky and clumps together into drops because of its cohesive properties, but chemistry and. Cohesive and adhesive forces. Why Is Water Cohesive And Adhesive.
From quizanglicists.z21.web.core.windows.net
Why Is Water Cohesive Why Is Water Cohesive And Adhesive This is why you can fill a glass of water just barely above the rim without. Why are cohesive and adhesive forces important for life? Provide examples of water’s cohesive and adhesive properties; Water is sticky and clumps together into drops because of its cohesive properties, but chemistry and. However, when water is filled to the tip of the cylinder,. Why Is Water Cohesive And Adhesive.
From www.slideserve.com
PPT Properties of Liquids PowerPoint Presentation, free download ID Why Is Water Cohesive And Adhesive However, when water is filled to the tip of the cylinder, the water level could maintain higher than the wall of the cylinder without pouring out resembling a concave down meniscus. Water molecules are very cohesive because of the molecule's polarity. Provide examples of water’s cohesive and adhesive properties; Cohesive and adhesive forces are important for the transport of water. Why Is Water Cohesive And Adhesive.
From www.slideserve.com
PPT Just plain old water! PowerPoint Presentation, free download ID Why Is Water Cohesive And Adhesive Water molecules are very cohesive because of the molecule's polarity. One property is cohesion, the tendency for water molecules to stick together. This is why you can fill a glass of water just barely above the rim without. Why are cohesive and adhesive forces important for life? Provide examples of water’s cohesive and adhesive properties; Cohesive and adhesive forces are. Why Is Water Cohesive And Adhesive.
From bio.libretexts.org
2.16 Water Cohesive and Adhesive Properties Biology LibreTexts Why Is Water Cohesive And Adhesive Cohesive and adhesive forces are important for the transport of water from the. However, when water is filled to the tip of the cylinder, the water level could maintain higher than the wall of the cylinder without pouring out resembling a concave down meniscus. Discuss the role of acids, bases, and buffers in homeostasis Explain why water is an excellent. Why Is Water Cohesive And Adhesive.
From www.slideserve.com
PPT Ch 11 States of Matter and Intermolecular Forces PowerPoint Why Is Water Cohesive And Adhesive One property is cohesion, the tendency for water molecules to stick together. Water is sticky and clumps together into drops because of its cohesive properties, but chemistry and. Provide examples of water’s cohesive and adhesive properties; Why are cohesive and adhesive forces important for life? Explain why water is an excellent solvent; Explain why water is an excellent solvent; This. Why Is Water Cohesive And Adhesive.
From www.slideserve.com
PPT Water Chemistry & Properties of Water PowerPoint Presentation Why Is Water Cohesive And Adhesive Why are cohesive and adhesive forces important for life? Cohesion allows substances to withstand rupture when placed under stress while adhesion is the attraction between water and other molecules. Discuss the role of acids, bases, and buffers in homeostasis Discuss the role of acids, bases, and buffers in homeostasis Explain why water is an excellent solvent; Cohesive and adhesive forces. Why Is Water Cohesive And Adhesive.
From www.slideserve.com
PPT Water and It’s properties PowerPoint Presentation, free download Why Is Water Cohesive And Adhesive Cohesion causes water to have surface tension, the property that. Explain why water is an excellent solvent; Provide examples of water’s cohesive and adhesive properties; Water molecules are very cohesive because of the molecule's polarity. Why are cohesive and adhesive forces important for life? Cohesion allows substances to withstand rupture when placed under stress while adhesion is the attraction between. Why Is Water Cohesive And Adhesive.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint Why Is Water Cohesive And Adhesive Explain why water is an excellent solvent; Water is sticky and clumps together into drops because of its cohesive properties, but chemistry and. Why are cohesive and adhesive forces important for life? Water molecules are very cohesive because of the molecule's polarity. Cohesion causes water to have surface tension, the property that. This is why you can fill a glass. Why Is Water Cohesive And Adhesive.
From www.animalia-life.club
Cohesion Of Water Diagram Why Is Water Cohesive And Adhesive Provide examples of water’s cohesive and adhesive properties; Cohesive and adhesive forces are important for the transport of water from the. Cohesion causes water to have surface tension, the property that. Discuss the role of acids, bases, and buffers in homeostasis Explain why water is an excellent solvent; Discuss the role of acids, bases, and buffers in homeostasis Explain why. Why Is Water Cohesive And Adhesive.
From www.slideserve.com
PPT Cohesion, Adhesion, and Surface Tension PowerPoint Presentation Why Is Water Cohesive And Adhesive Cohesion causes water to have surface tension, the property that. Provide examples of water’s cohesive and adhesive properties; Cohesive and adhesive forces are important for the transport of water from the. Cohesion allows substances to withstand rupture when placed under stress while adhesion is the attraction between water and other molecules. Water is sticky and clumps together into drops because. Why Is Water Cohesive And Adhesive.
From www.animalia-life.club
Adhesion Of Water Diagram Why Is Water Cohesive And Adhesive One property is cohesion, the tendency for water molecules to stick together. Provide examples of water’s cohesive and adhesive properties; Discuss the role of acids, bases, and buffers in homeostasis However, when water is filled to the tip of the cylinder, the water level could maintain higher than the wall of the cylinder without pouring out resembling a concave down. Why Is Water Cohesive And Adhesive.
From www.expii.com
Cohesion and Adhesion (Water) — Properties & Examples Expii Why Is Water Cohesive And Adhesive Discuss the role of acids, bases, and buffers in homeostasis Why are cohesive and adhesive forces important for life? Water molecules are very cohesive because of the molecule's polarity. Explain why water is an excellent solvent; Cohesive and adhesive forces are important for the transport of water from the. One property is cohesion, the tendency for water molecules to stick. Why Is Water Cohesive And Adhesive.
From ar.inspiredpencil.com
Cohesion And Adhesion Examples Why Is Water Cohesive And Adhesive Discuss the role of acids, bases, and buffers in homeostasis Cohesion allows substances to withstand rupture when placed under stress while adhesion is the attraction between water and other molecules. Water molecules are very cohesive because of the molecule's polarity. Discuss the role of acids, bases, and buffers in homeostasis However, when water is filled to the tip of the. Why Is Water Cohesive And Adhesive.
From www.pinterest.com
Difference Between Cohesion and Adhesion Definition, Relationship Why Is Water Cohesive And Adhesive Explain why water is an excellent solvent; Cohesion causes water to have surface tension, the property that. Discuss the role of acids, bases, and buffers in homeostasis Water is sticky and clumps together into drops because of its cohesive properties, but chemistry and. This is why you can fill a glass of water just barely above the rim without. Explain. Why Is Water Cohesive And Adhesive.
From mappingmemories.ca
erección Murciélago Hacer las tareas domésticas cohesion and adhesion Why Is Water Cohesive And Adhesive Provide examples of water’s cohesive and adhesive properties; Cohesive and adhesive forces are important for the transport of water from the. This is why you can fill a glass of water just barely above the rim without. Cohesion causes water to have surface tension, the property that. Water is sticky and clumps together into drops because of its cohesive properties,. Why Is Water Cohesive And Adhesive.
From www.geeksforgeeks.org
Difference between Adhesion and Cohesion Why Is Water Cohesive And Adhesive This is why you can fill a glass of water just barely above the rim without. Cohesion causes water to have surface tension, the property that. One property is cohesion, the tendency for water molecules to stick together. Discuss the role of acids, bases, and buffers in homeostasis Discuss the role of acids, bases, and buffers in homeostasis However, when. Why Is Water Cohesive And Adhesive.
From www.animalia-life.club
Adhesion Of Water Meniscus Why Is Water Cohesive And Adhesive Discuss the role of acids, bases, and buffers in homeostasis Explain why water is an excellent solvent; Water molecules are very cohesive because of the molecule's polarity. This is why you can fill a glass of water just barely above the rim without. Provide examples of water’s cohesive and adhesive properties; One property is cohesion, the tendency for water molecules. Why Is Water Cohesive And Adhesive.
From bio.libretexts.org
4.5.1.3 CohesionTension Theory Biology LibreTexts Why Is Water Cohesive And Adhesive However, when water is filled to the tip of the cylinder, the water level could maintain higher than the wall of the cylinder without pouring out resembling a concave down meniscus. Explain why water is an excellent solvent; Why are cohesive and adhesive forces important for life? Provide examples of water’s cohesive and adhesive properties; Discuss the role of acids,. Why Is Water Cohesive And Adhesive.
From www.slideserve.com
PPT Chapter 9 Intermolecular Forces, Liquids, and Solids PowerPoint Why Is Water Cohesive And Adhesive Water is sticky and clumps together into drops because of its cohesive properties, but chemistry and. Cohesion causes water to have surface tension, the property that. Discuss the role of acids, bases, and buffers in homeostasis This is why you can fill a glass of water just barely above the rim without. Discuss the role of acids, bases, and buffers. Why Is Water Cohesive And Adhesive.
From www.slideshare.net
Chapter 9 Transport in Plants Lesson 3 The 3 mechanisms in water tr… Why Is Water Cohesive And Adhesive Explain why water is an excellent solvent; Provide examples of water’s cohesive and adhesive properties; Water is sticky and clumps together into drops because of its cohesive properties, but chemistry and. Discuss the role of acids, bases, and buffers in homeostasis Why are cohesive and adhesive forces important for life? Cohesive and adhesive forces are important for the transport of. Why Is Water Cohesive And Adhesive.
From www.slideserve.com
PPT Understanding Water PowerPoint Presentation, free download ID Why Is Water Cohesive And Adhesive Water molecules are very cohesive because of the molecule's polarity. Explain why water is an excellent solvent; Discuss the role of acids, bases, and buffers in homeostasis Provide examples of water’s cohesive and adhesive properties; Why are cohesive and adhesive forces important for life? Water is sticky and clumps together into drops because of its cohesive properties, but chemistry and.. Why Is Water Cohesive And Adhesive.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation ID2370697 Why Is Water Cohesive And Adhesive Discuss the role of acids, bases, and buffers in homeostasis Explain why water is an excellent solvent; Discuss the role of acids, bases, and buffers in homeostasis This is why you can fill a glass of water just barely above the rim without. Provide examples of water’s cohesive and adhesive properties; Cohesion causes water to have surface tension, the property. Why Is Water Cohesive And Adhesive.
From www.vrogue.co
11 Biology Cohesive And Adhesive Forces In Water Comp vrogue.co Why Is Water Cohesive And Adhesive Why are cohesive and adhesive forces important for life? Water molecules are very cohesive because of the molecule's polarity. Cohesive and adhesive forces are important for the transport of water from the. Cohesion allows substances to withstand rupture when placed under stress while adhesion is the attraction between water and other molecules. Explain why water is an excellent solvent; However,. Why Is Water Cohesive And Adhesive.
From www.scienceabc.com
Why Does Water Feel Like Concrete When You Belly Flop Into It? » ScienceABC Why Is Water Cohesive And Adhesive Provide examples of water’s cohesive and adhesive properties; Discuss the role of acids, bases, and buffers in homeostasis Cohesion causes water to have surface tension, the property that. Water is sticky and clumps together into drops because of its cohesive properties, but chemistry and. However, when water is filled to the tip of the cylinder, the water level could maintain. Why Is Water Cohesive And Adhesive.
From www.slideserve.com
PPT Properties of Liquids PowerPoint Presentation, free download ID Why Is Water Cohesive And Adhesive Cohesive and adhesive forces are important for the transport of water from the. Why are cohesive and adhesive forces important for life? Explain why water is an excellent solvent; Provide examples of water’s cohesive and adhesive properties; Discuss the role of acids, bases, and buffers in homeostasis This is why you can fill a glass of water just barely above. Why Is Water Cohesive And Adhesive.
From quizizz.com
Properties of Water Quizizz Why Is Water Cohesive And Adhesive Provide examples of water’s cohesive and adhesive properties; Why are cohesive and adhesive forces important for life? However, when water is filled to the tip of the cylinder, the water level could maintain higher than the wall of the cylinder without pouring out resembling a concave down meniscus. One property is cohesion, the tendency for water molecules to stick together.. Why Is Water Cohesive And Adhesive.
From www.expii.com
Cohesion and Adhesion (Water) — Properties & Examples Expii Why Is Water Cohesive And Adhesive Discuss the role of acids, bases, and buffers in homeostasis Cohesion allows substances to withstand rupture when placed under stress while adhesion is the attraction between water and other molecules. Provide examples of water’s cohesive and adhesive properties; Provide examples of water’s cohesive and adhesive properties; Cohesion causes water to have surface tension, the property that. Discuss the role of. Why Is Water Cohesive And Adhesive.
From www.youtube.com
Cohesive and Adhesive Forces YouTube Why Is Water Cohesive And Adhesive Water molecules are very cohesive because of the molecule's polarity. Discuss the role of acids, bases, and buffers in homeostasis Water is sticky and clumps together into drops because of its cohesive properties, but chemistry and. This is why you can fill a glass of water just barely above the rim without. Why are cohesive and adhesive forces important for. Why Is Water Cohesive And Adhesive.
From ar.inspiredpencil.com
Cohesion And Adhesion Why Is Water Cohesive And Adhesive Water molecules are very cohesive because of the molecule's polarity. Water is sticky and clumps together into drops because of its cohesive properties, but chemistry and. This is why you can fill a glass of water just barely above the rim without. Cohesion allows substances to withstand rupture when placed under stress while adhesion is the attraction between water and. Why Is Water Cohesive And Adhesive.
From www.slideserve.com
PPT Water (H2O) PowerPoint Presentation, free download ID1700557 Why Is Water Cohesive And Adhesive Explain why water is an excellent solvent; Water molecules are very cohesive because of the molecule's polarity. Cohesion allows substances to withstand rupture when placed under stress while adhesion is the attraction between water and other molecules. This is why you can fill a glass of water just barely above the rim without. Provide examples of water’s cohesive and adhesive. Why Is Water Cohesive And Adhesive.
From www.vrogue.co
Cohesion Of Water Diagram vrogue.co Why Is Water Cohesive And Adhesive Water molecules are very cohesive because of the molecule's polarity. Discuss the role of acids, bases, and buffers in homeostasis Explain why water is an excellent solvent; Provide examples of water’s cohesive and adhesive properties; This is why you can fill a glass of water just barely above the rim without. Discuss the role of acids, bases, and buffers in. Why Is Water Cohesive And Adhesive.