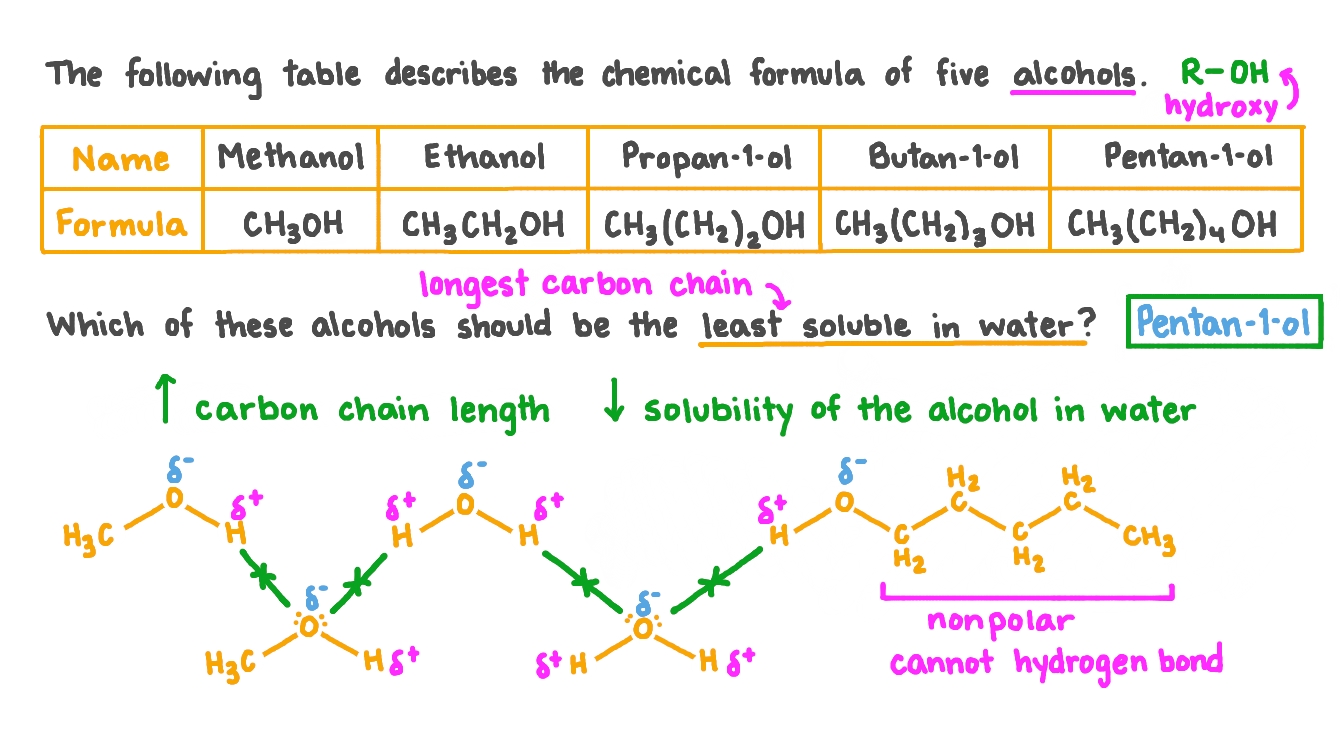
How Does Alcohol Dissolve In Water . Solubility of alcohols is therefore determined by the stronger of the two forces. Water is a terrible solvent for nonpolar hydrocarbon molecules: Alcohols, like water, are both weak bases and weak acids. Because of the strength of the attraction of the oh group, first. Next, you try a series of increasingly large alcohol compounds,. Alcohols, as might be expected, have properties between the extremes of hydrocarbons and water. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. The solubility of alcohols in water depends on the size of the alcohol molecule and the presence of hydrophilic functional groups. When the hydrocarbon chain is short, the. We frequently find that the borderline of solubility in a family of organic compounds occurs at four or five carbon
from klalyxthl.blob.core.windows.net
When the hydrocarbon chain is short, the. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. Alcohols, as might be expected, have properties between the extremes of hydrocarbons and water. Solubility of alcohols is therefore determined by the stronger of the two forces. We frequently find that the borderline of solubility in a family of organic compounds occurs at four or five carbon The solubility of alcohols in water depends on the size of the alcohol molecule and the presence of hydrophilic functional groups. Water is a terrible solvent for nonpolar hydrocarbon molecules: Because of the strength of the attraction of the oh group, first. Next, you try a series of increasingly large alcohol compounds,. Alcohols, like water, are both weak bases and weak acids.
Does Denatured Alcohol Dissolve In Water at Josephine Jacobs blog
How Does Alcohol Dissolve In Water In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. Alcohols, as might be expected, have properties between the extremes of hydrocarbons and water. Next, you try a series of increasingly large alcohol compounds,. Water is a terrible solvent for nonpolar hydrocarbon molecules: In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. We frequently find that the borderline of solubility in a family of organic compounds occurs at four or five carbon Alcohols, like water, are both weak bases and weak acids. Because of the strength of the attraction of the oh group, first. When the hydrocarbon chain is short, the. Solubility of alcohols is therefore determined by the stronger of the two forces. The solubility of alcohols in water depends on the size of the alcohol molecule and the presence of hydrophilic functional groups.
From sciencemsqblog8.blogspot.com
Science8 Semester 2,Chapter 4 Mixtures How Does Alcohol Dissolve In Water Alcohols, like water, are both weak bases and weak acids. Alcohols, as might be expected, have properties between the extremes of hydrocarbons and water. We frequently find that the borderline of solubility in a family of organic compounds occurs at four or five carbon When the hydrocarbon chain is short, the. Water is a terrible solvent for nonpolar hydrocarbon molecules:. How Does Alcohol Dissolve In Water.
From addictadvice.com
Why is Polyvinyl Alcohol Soluble in Water? How Does Alcohol Dissolve In Water Solubility of alcohols is therefore determined by the stronger of the two forces. We frequently find that the borderline of solubility in a family of organic compounds occurs at four or five carbon Next, you try a series of increasingly large alcohol compounds,. Water is a terrible solvent for nonpolar hydrocarbon molecules: Alcohols, like water, are both weak bases and. How Does Alcohol Dissolve In Water.
From www.youtube.com
Why Does This Powder Only Dissolve In Cold Water? YouTube How Does Alcohol Dissolve In Water Solubility of alcohols is therefore determined by the stronger of the two forces. Because of the strength of the attraction of the oh group, first. Alcohols, like water, are both weak bases and weak acids. When the hydrocarbon chain is short, the. Water is a terrible solvent for nonpolar hydrocarbon molecules: Next, you try a series of increasingly large alcohol. How Does Alcohol Dissolve In Water.
From www.youtube.com
Can Liquids Dissolve in Water? YouTube How Does Alcohol Dissolve In Water Because of the strength of the attraction of the oh group, first. When the hydrocarbon chain is short, the. Solubility of alcohols is therefore determined by the stronger of the two forces. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. The solubility of alcohols in water depends on the size of the alcohol molecule and the. How Does Alcohol Dissolve In Water.
From www.slideserve.com
PPT Classification of Matter PowerPoint Presentation, free download How Does Alcohol Dissolve In Water The solubility of alcohols in water depends on the size of the alcohol molecule and the presence of hydrophilic functional groups. When the hydrocarbon chain is short, the. Solubility of alcohols is therefore determined by the stronger of the two forces. Next, you try a series of increasingly large alcohol compounds,. Because of the strength of the attraction of the. How Does Alcohol Dissolve In Water.
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids How Does Alcohol Dissolve In Water Alcohols, like water, are both weak bases and weak acids. Because of the strength of the attraction of the oh group, first. Next, you try a series of increasingly large alcohol compounds,. We frequently find that the borderline of solubility in a family of organic compounds occurs at four or five carbon Water is a terrible solvent for nonpolar hydrocarbon. How Does Alcohol Dissolve In Water.
From klasrisase.github.io
What Does Cazadores Mean In Spanish Burger King Manager Confronts How Does Alcohol Dissolve In Water The solubility of alcohols in water depends on the size of the alcohol molecule and the presence of hydrophilic functional groups. When the hydrocarbon chain is short, the. Because of the strength of the attraction of the oh group, first. Alcohols, like water, are both weak bases and weak acids. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen. How Does Alcohol Dissolve In Water.
From loegaqddj.blob.core.windows.net
Alcohol Dissolve In Water Or Not at Monica blog How Does Alcohol Dissolve In Water Water is a terrible solvent for nonpolar hydrocarbon molecules: The solubility of alcohols in water depends on the size of the alcohol molecule and the presence of hydrophilic functional groups. Because of the strength of the attraction of the oh group, first. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. Solubility of alcohols is therefore determined. How Does Alcohol Dissolve In Water.
From exonfafiq.blob.core.windows.net
Does Salt Dissolve In Room Temperature Water at Peter Allen blog How Does Alcohol Dissolve In Water Because of the strength of the attraction of the oh group, first. When the hydrocarbon chain is short, the. Alcohols, as might be expected, have properties between the extremes of hydrocarbons and water. Next, you try a series of increasingly large alcohol compounds,. Alcohols, like water, are both weak bases and weak acids. The solubility of alcohols in water depends. How Does Alcohol Dissolve In Water.
From www.youtube.com
Equation for LiOH + H2O (Lithium hydroxide + Water) YouTube How Does Alcohol Dissolve In Water In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. Alcohols, like water, are both weak bases and weak acids. Alcohols, as might be expected, have properties between the extremes of hydrocarbons and water. Water is a terrible solvent for nonpolar hydrocarbon molecules: Next, you try a series of increasingly large alcohol compounds,. Solubility of alcohols is therefore. How Does Alcohol Dissolve In Water.
From klalyxthl.blob.core.windows.net
Does Denatured Alcohol Dissolve In Water at Josephine Jacobs blog How Does Alcohol Dissolve In Water The solubility of alcohols in water depends on the size of the alcohol molecule and the presence of hydrophilic functional groups. Because of the strength of the attraction of the oh group, first. We frequently find that the borderline of solubility in a family of organic compounds occurs at four or five carbon Solubility of alcohols is therefore determined by. How Does Alcohol Dissolve In Water.
From www.youtube.com
Water, Alcohol, Oil YouTube How Does Alcohol Dissolve In Water When the hydrocarbon chain is short, the. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. Solubility of alcohols is therefore determined by the stronger of the two forces. Alcohols, like water, are both weak bases and weak acids. Alcohols, as might be expected, have properties between the extremes of hydrocarbons and water. We frequently find that. How Does Alcohol Dissolve In Water.
From dxodwjtkf.blob.core.windows.net
What Happens When You Put Apple In Salt Water at Leonard Rogers blog How Does Alcohol Dissolve In Water The solubility of alcohols in water depends on the size of the alcohol molecule and the presence of hydrophilic functional groups. Because of the strength of the attraction of the oh group, first. Water is a terrible solvent for nonpolar hydrocarbon molecules: Alcohols, as might be expected, have properties between the extremes of hydrocarbons and water. When the hydrocarbon chain. How Does Alcohol Dissolve In Water.
From gluethings.com
Does rubbing alcohol dissolve epoxy? Glue Things How Does Alcohol Dissolve In Water Alcohols, as might be expected, have properties between the extremes of hydrocarbons and water. The solubility of alcohols in water depends on the size of the alcohol molecule and the presence of hydrophilic functional groups. Solubility of alcohols is therefore determined by the stronger of the two forces. We frequently find that the borderline of solubility in a family of. How Does Alcohol Dissolve In Water.
From byjus.com
Perform an activity to find out how to dissolve a solid in a liquid? How Does Alcohol Dissolve In Water Solubility of alcohols is therefore determined by the stronger of the two forces. When the hydrocarbon chain is short, the. We frequently find that the borderline of solubility in a family of organic compounds occurs at four or five carbon Alcohols, like water, are both weak bases and weak acids. Next, you try a series of increasingly large alcohol compounds,.. How Does Alcohol Dissolve In Water.
From www.youtube.com
Experiment3 Isolation of egg albumin from egg white YouTube How Does Alcohol Dissolve In Water Solubility of alcohols is therefore determined by the stronger of the two forces. Water is a terrible solvent for nonpolar hydrocarbon molecules: When the hydrocarbon chain is short, the. Because of the strength of the attraction of the oh group, first. The solubility of alcohols in water depends on the size of the alcohol molecule and the presence of hydrophilic. How Does Alcohol Dissolve In Water.
From www.acs.org
Lesson 5.3 Why Does Water Dissolve Salt? American Chemical Society How Does Alcohol Dissolve In Water We frequently find that the borderline of solubility in a family of organic compounds occurs at four or five carbon When the hydrocarbon chain is short, the. Next, you try a series of increasingly large alcohol compounds,. Alcohols, like water, are both weak bases and weak acids. Because of the strength of the attraction of the oh group, first. Water. How Does Alcohol Dissolve In Water.
From www.koyuncusalt.com
Why Does Salt Dissolve In Water? How to Separate Them Back? Salt How Does Alcohol Dissolve In Water Because of the strength of the attraction of the oh group, first. Alcohols, like water, are both weak bases and weak acids. Water is a terrible solvent for nonpolar hydrocarbon molecules: Alcohols, as might be expected, have properties between the extremes of hydrocarbons and water. Solubility of alcohols is therefore determined by the stronger of the two forces. When the. How Does Alcohol Dissolve In Water.
From scholarsark.com
How do you seperate alcohol from water Scholars Ark How Does Alcohol Dissolve In Water We frequently find that the borderline of solubility in a family of organic compounds occurs at four or five carbon Alcohols, like water, are both weak bases and weak acids. Solubility of alcohols is therefore determined by the stronger of the two forces. The solubility of alcohols in water depends on the size of the alcohol molecule and the presence. How Does Alcohol Dissolve In Water.
From builderbold.com
Does salt dissolve in alcohol? BuilderBold How Does Alcohol Dissolve In Water Next, you try a series of increasingly large alcohol compounds,. When the hydrocarbon chain is short, the. Alcohols, like water, are both weak bases and weak acids. Because of the strength of the attraction of the oh group, first. Solubility of alcohols is therefore determined by the stronger of the two forces. We frequently find that the borderline of solubility. How Does Alcohol Dissolve In Water.
From loegaqddj.blob.core.windows.net
Alcohol Dissolve In Water Or Not at Monica blog How Does Alcohol Dissolve In Water We frequently find that the borderline of solubility in a family of organic compounds occurs at four or five carbon Next, you try a series of increasingly large alcohol compounds,. The solubility of alcohols in water depends on the size of the alcohol molecule and the presence of hydrophilic functional groups. In aqueous solutions, alcohols dissociate slightly by donating a. How Does Alcohol Dissolve In Water.
From yesdirt.com
Does Alcohol Dissolve in Water? (ANSWERED) Yes Dirt How Does Alcohol Dissolve In Water When the hydrocarbon chain is short, the. We frequently find that the borderline of solubility in a family of organic compounds occurs at four or five carbon Water is a terrible solvent for nonpolar hydrocarbon molecules: Next, you try a series of increasingly large alcohol compounds,. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. Solubility of. How Does Alcohol Dissolve In Water.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID2687396 How Does Alcohol Dissolve In Water Alcohols, like water, are both weak bases and weak acids. When the hydrocarbon chain is short, the. We frequently find that the borderline of solubility in a family of organic compounds occurs at four or five carbon The solubility of alcohols in water depends on the size of the alcohol molecule and the presence of hydrophilic functional groups. Water is. How Does Alcohol Dissolve In Water.
From pressbooks.bccampus.ca
6.3 AcidBase Reactions CHEM 1114 Introduction to Chemistry How Does Alcohol Dissolve In Water When the hydrocarbon chain is short, the. Solubility of alcohols is therefore determined by the stronger of the two forces. Because of the strength of the attraction of the oh group, first. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. We frequently find that the borderline of solubility in a family of organic compounds occurs at. How Does Alcohol Dissolve In Water.
From www.nagwa.com
Question Video Explaining Why the Volume of an Alcohol and Water How Does Alcohol Dissolve In Water When the hydrocarbon chain is short, the. Next, you try a series of increasingly large alcohol compounds,. Solubility of alcohols is therefore determined by the stronger of the two forces. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. Because of the strength of the attraction of the oh group, first. We frequently find that the borderline. How Does Alcohol Dissolve In Water.
From klalyxthl.blob.core.windows.net
Does Denatured Alcohol Dissolve In Water at Josephine Jacobs blog How Does Alcohol Dissolve In Water Solubility of alcohols is therefore determined by the stronger of the two forces. Next, you try a series of increasingly large alcohol compounds,. The solubility of alcohols in water depends on the size of the alcohol molecule and the presence of hydrophilic functional groups. Because of the strength of the attraction of the oh group, first. When the hydrocarbon chain. How Does Alcohol Dissolve In Water.
From www.vecteezy.com
Dissolving science experiment with sugar dissolve in water 3333021 How Does Alcohol Dissolve In Water Alcohols, as might be expected, have properties between the extremes of hydrocarbons and water. We frequently find that the borderline of solubility in a family of organic compounds occurs at four or five carbon Alcohols, like water, are both weak bases and weak acids. Next, you try a series of increasingly large alcohol compounds,. In aqueous solutions, alcohols dissociate slightly. How Does Alcohol Dissolve In Water.
From klalyxthl.blob.core.windows.net
Does Denatured Alcohol Dissolve In Water at Josephine Jacobs blog How Does Alcohol Dissolve In Water The solubility of alcohols in water depends on the size of the alcohol molecule and the presence of hydrophilic functional groups. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. Alcohols, as might be expected, have properties between the extremes of hydrocarbons and water. Alcohols, like water, are both weak bases and weak acids. We frequently find. How Does Alcohol Dissolve In Water.
From yesdirt.com
Does Sugar Dissolve in Alcohol? (ANSWERED) Yes Dirt How Does Alcohol Dissolve In Water Alcohols, as might be expected, have properties between the extremes of hydrocarbons and water. Water is a terrible solvent for nonpolar hydrocarbon molecules: Solubility of alcohols is therefore determined by the stronger of the two forces. The solubility of alcohols in water depends on the size of the alcohol molecule and the presence of hydrophilic functional groups. Because of the. How Does Alcohol Dissolve In Water.
From www.vecteezy.com
Dissolving science experiment with sugar dissolve in water 3188660 How Does Alcohol Dissolve In Water Solubility of alcohols is therefore determined by the stronger of the two forces. Water is a terrible solvent for nonpolar hydrocarbon molecules: Because of the strength of the attraction of the oh group, first. When the hydrocarbon chain is short, the. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. Next, you try a series of increasingly. How Does Alcohol Dissolve In Water.
From www.vectorstock.com
How does sodium chloride nacl dissolve in water Vector Image How Does Alcohol Dissolve In Water When the hydrocarbon chain is short, the. Next, you try a series of increasingly large alcohol compounds,. Water is a terrible solvent for nonpolar hydrocarbon molecules: Alcohols, as might be expected, have properties between the extremes of hydrocarbons and water. The solubility of alcohols in water depends on the size of the alcohol molecule and the presence of hydrophilic functional. How Does Alcohol Dissolve In Water.
From www.youtube.com
How To Separate Alcohol And Water YouTube How Does Alcohol Dissolve In Water We frequently find that the borderline of solubility in a family of organic compounds occurs at four or five carbon When the hydrocarbon chain is short, the. Solubility of alcohols is therefore determined by the stronger of the two forces. Because of the strength of the attraction of the oh group, first. Water is a terrible solvent for nonpolar hydrocarbon. How Does Alcohol Dissolve In Water.
From sierraukung.blogspot.com
Discussion Of Absorbing Carbon Dioxide In Water How Does Alcohol Dissolve In Water We frequently find that the borderline of solubility in a family of organic compounds occurs at four or five carbon Because of the strength of the attraction of the oh group, first. In aqueous solutions, alcohols dissociate slightly by donating a hydrogen to water. Alcohols, like water, are both weak bases and weak acids. Solubility of alcohols is therefore determined. How Does Alcohol Dissolve In Water.
From saylordotorg.github.io
Physical Properties of Alcohols How Does Alcohol Dissolve In Water Alcohols, like water, are both weak bases and weak acids. We frequently find that the borderline of solubility in a family of organic compounds occurs at four or five carbon The solubility of alcohols in water depends on the size of the alcohol molecule and the presence of hydrophilic functional groups. When the hydrocarbon chain is short, the. Alcohols, as. How Does Alcohol Dissolve In Water.
From www.youtube.com
Polyvinyl alcohol PVA dissolving in water timelapse YouTube How Does Alcohol Dissolve In Water Alcohols, like water, are both weak bases and weak acids. Because of the strength of the attraction of the oh group, first. Solubility of alcohols is therefore determined by the stronger of the two forces. Next, you try a series of increasingly large alcohol compounds,. Water is a terrible solvent for nonpolar hydrocarbon molecules: The solubility of alcohols in water. How Does Alcohol Dissolve In Water.