Lab 6 Acid Base Titration . there are two basic types of acid base titrations, indicator and potentiometric. titration is an analytical quantitative technique used to determine the concentration of a solute; The analyte (titrand) is the solution with an unknown molarity. use the sodium hydroxide, naoh, solution that you standardized in lab 6 as your base of known concentration. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. The reagent (titrant) is the solution with a known molarity that will react with the analyte. By thomas cahill, arizona state university, new college of interdisciplinary arts and sciences.
from www.chegg.com
It is based on the neutralization reaction, where an acid and a base react to form water and a salt. The reagent (titrant) is the solution with a known molarity that will react with the analyte. there are two basic types of acid base titrations, indicator and potentiometric. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. titration is an analytical quantitative technique used to determine the concentration of a solute; The analyte (titrand) is the solution with an unknown molarity. use the sodium hydroxide, naoh, solution that you standardized in lab 6 as your base of known concentration. By thomas cahill, arizona state university, new college of interdisciplinary arts and sciences.
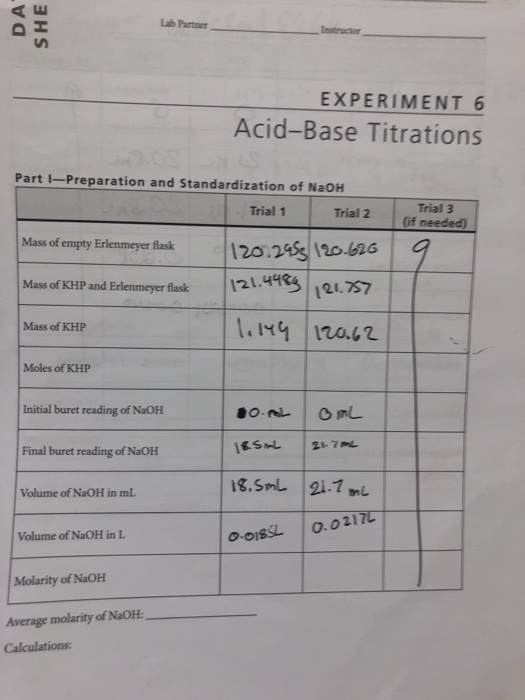
Solved Lab Partner Instrucior EXPERIMENT 6 AcidBase
Lab 6 Acid Base Titration use the sodium hydroxide, naoh, solution that you standardized in lab 6 as your base of known concentration. there are two basic types of acid base titrations, indicator and potentiometric. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. use the sodium hydroxide, naoh, solution that you standardized in lab 6 as your base of known concentration. titration is an analytical quantitative technique used to determine the concentration of a solute; The reagent (titrant) is the solution with a known molarity that will react with the analyte. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. The analyte (titrand) is the solution with an unknown molarity. By thomas cahill, arizona state university, new college of interdisciplinary arts and sciences.
From mungfali.com
Acid Base Titration Lab Lab 6 Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. use the sodium hydroxide, naoh, solution that you standardized in lab 6 as your base of known concentration. there are two basic types of acid base titrations, indicator and potentiometric. It is based on the neutralization reaction, where an acid and a base react to form water and. Lab 6 Acid Base Titration.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Lab 6 Acid Base Titration there are two basic types of acid base titrations, indicator and potentiometric. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. titration is an analytical quantitative technique used to determine the concentration of a solute; The analyte (titrand) is the solution with an unknown molarity. use the. Lab 6 Acid Base Titration.
From exoxuekni.blob.core.windows.net
Acid Base Titration Uses at Jennifer Baird blog Lab 6 Acid Base Titration By thomas cahill, arizona state university, new college of interdisciplinary arts and sciences. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. titration is an analytical quantitative technique used to determine the concentration of a solute; The analyte (titrand) is the solution with an unknown molarity. The reagent. Lab 6 Acid Base Titration.
From www.studypool.com
SOLUTION Lab 6 acid base titrations Studypool Lab 6 Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. there are two basic types of acid base titrations, indicator and potentiometric. By thomas cahill, arizona state university, new college of interdisciplinary arts and sciences. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. study with quizlet. Lab 6 Acid Base Titration.
From www.youtube.com
Acid Base Titration Experiment Acid base Titration Practical and Lab 6 Acid Base Titration The reagent (titrant) is the solution with a known molarity that will react with the analyte. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. titration is an analytical quantitative technique used to determine the concentration of a solute; The analyte (titrand) is the solution with an unknown. Lab 6 Acid Base Titration.
From www.chegg.com
Solved LABORATORY 6 ACIDBASE TITRATION INTRODUCTION A Lab 6 Acid Base Titration titration is an analytical quantitative technique used to determine the concentration of a solute; there are two basic types of acid base titrations, indicator and potentiometric. The reagent (titrant) is the solution with a known molarity that will react with the analyte. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic,. Lab 6 Acid Base Titration.
From mungfali.com
Acid Base Titration Lab Lab 6 Acid Base Titration It is based on the neutralization reaction, where an acid and a base react to form water and a salt. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. use the sodium hydroxide, naoh, solution that you standardized in lab 6 as your base of known concentration. The analyte. Lab 6 Acid Base Titration.
From moniquearesmeza.blogspot.com
Acid Base Titration Lab Report Answers MoniquearesMeza Lab 6 Acid Base Titration use the sodium hydroxide, naoh, solution that you standardized in lab 6 as your base of known concentration. By thomas cahill, arizona state university, new college of interdisciplinary arts and sciences. The analyte (titrand) is the solution with an unknown molarity. It is based on the neutralization reaction, where an acid and a base react to form water and. Lab 6 Acid Base Titration.
From mungfali.com
Acid Base Titration Lab Procedure Lab 6 Acid Base Titration titration is an analytical quantitative technique used to determine the concentration of a solute; use the sodium hydroxide, naoh, solution that you standardized in lab 6 as your base of known concentration. The reagent (titrant) is the solution with a known molarity that will react with the analyte. It is based on the neutralization reaction, where an acid. Lab 6 Acid Base Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Lab 6 Acid Base Titration study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. use the sodium hydroxide, naoh, solution that you standardized in lab 6 as your base of known concentration. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. titration. Lab 6 Acid Base Titration.
From www.studypool.com
SOLUTION Lab 6 acid base titrations Studypool Lab 6 Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. By thomas cahill, arizona state university, new college of interdisciplinary arts and sciences. It is based on the neutralization reaction, where an acid and a base react to form water and. Lab 6 Acid Base Titration.
From dxokhzpxh.blob.core.windows.net
Aim Of Acid Base Titration Experiment at Ebony Mooney blog Lab 6 Acid Base Titration By thomas cahill, arizona state university, new college of interdisciplinary arts and sciences. titration is an analytical quantitative technique used to determine the concentration of a solute; use the sodium hydroxide, naoh, solution that you standardized in lab 6 as your base of known concentration. there are two basic types of acid base titrations, indicator and potentiometric.. Lab 6 Acid Base Titration.
From www.studypool.com
SOLUTION Lab 6 acid base titrations Studypool Lab 6 Acid Base Titration It is based on the neutralization reaction, where an acid and a base react to form water and a salt. The reagent (titrant) is the solution with a known molarity that will react with the analyte. By thomas cahill, arizona state university, new college of interdisciplinary arts and sciences. there are two basic types of acid base titrations, indicator. Lab 6 Acid Base Titration.
From www.vrogue.co
Acid Base Titration Experiment And Phases Of Color Ch vrogue.co Lab 6 Acid Base Titration It is based on the neutralization reaction, where an acid and a base react to form water and a salt. The analyte (titrand) is the solution with an unknown molarity. By thomas cahill, arizona state university, new college of interdisciplinary arts and sciences. The reagent (titrant) is the solution with a known molarity that will react with the analyte. . Lab 6 Acid Base Titration.
From www.chegg.com
Solved LABORATORY 6 ACIDBASE TITRATION INTRODUCTION A Lab 6 Acid Base Titration It is based on the neutralization reaction, where an acid and a base react to form water and a salt. titration is an analytical quantitative technique used to determine the concentration of a solute; study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. The reagent (titrant) is the solution. Lab 6 Acid Base Titration.
From mungfali.com
Acid Base Titration Lab Lab 6 Acid Base Titration there are two basic types of acid base titrations, indicator and potentiometric. titration is an analytical quantitative technique used to determine the concentration of a solute; use the sodium hydroxide, naoh, solution that you standardized in lab 6 as your base of known concentration. The reagent (titrant) is the solution with a known molarity that will react. Lab 6 Acid Base Titration.
From studylib.net
Lab 6 AcidBase Titration Lab 6 Acid Base Titration The reagent (titrant) is the solution with a known molarity that will react with the analyte. there are two basic types of acid base titrations, indicator and potentiometric. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. By thomas cahill, arizona state university, new college of interdisciplinary arts. Lab 6 Acid Base Titration.
From mavink.com
Acid Base Titration Lab Procedure Lab 6 Acid Base Titration study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. The analyte (titrand) is the solution with an unknown molarity. use the sodium hydroxide, naoh, solution that you standardized in lab 6 as your base of known concentration. It is based on the neutralization reaction, where an acid and a. Lab 6 Acid Base Titration.
From www.studypool.com
SOLUTION Acid base titration chemistry lab part 1 theory Lab 6 Acid Base Titration titration is an analytical quantitative technique used to determine the concentration of a solute; study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. By thomas cahill, arizona state university, new college of interdisciplinary arts and sciences. It is based on the neutralization reaction, where an acid and a base. Lab 6 Acid Base Titration.
From www.scribd.com
Lab Report Acid Base PDF Titration Chemistry Lab 6 Acid Base Titration titration is an analytical quantitative technique used to determine the concentration of a solute; By thomas cahill, arizona state university, new college of interdisciplinary arts and sciences. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. It is based on the neutralization reaction, where an acid and a base. Lab 6 Acid Base Titration.
From vdocuments.mx
Lab Session 7, Experiment 6 AcidBase Titration [Download PDF] Lab 6 Acid Base Titration By thomas cahill, arizona state university, new college of interdisciplinary arts and sciences. The analyte (titrand) is the solution with an unknown molarity. titration is an analytical quantitative technique used to determine the concentration of a solute; study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. use the. Lab 6 Acid Base Titration.
From exoxjybnj.blob.core.windows.net
Acid Base Titration Lab Introduction at Nichole Brumback blog Lab 6 Acid Base Titration The reagent (titrant) is the solution with a known molarity that will react with the analyte. there are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the solution with an unknown molarity. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. use. Lab 6 Acid Base Titration.
From www.chegg.com
Solved Lab Partner Instrucior EXPERIMENT 6 AcidBase Lab 6 Acid Base Titration use the sodium hydroxide, naoh, solution that you standardized in lab 6 as your base of known concentration. The reagent (titrant) is the solution with a known molarity that will react with the analyte. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. The analyte (titrand) is the solution. Lab 6 Acid Base Titration.
From www.docsity.com
Acid Base Titrations Lab Report General Chemistry Lab CHEM 1045 Lab 6 Acid Base Titration use the sodium hydroxide, naoh, solution that you standardized in lab 6 as your base of known concentration. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. The analyte (titrand) is the solution with an unknown molarity. titration is an analytical quantitative technique used to determine the concentration. Lab 6 Acid Base Titration.
From mungfali.com
Acid Base Titration Lab Lab 6 Acid Base Titration use the sodium hydroxide, naoh, solution that you standardized in lab 6 as your base of known concentration. The analyte (titrand) is the solution with an unknown molarity. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. The reagent (titrant) is the solution with a known molarity that. Lab 6 Acid Base Titration.
From www.youtube.com
Lab Demonstration Acid Base Titration. YouTube Lab 6 Acid Base Titration use the sodium hydroxide, naoh, solution that you standardized in lab 6 as your base of known concentration. The analyte (titrand) is the solution with an unknown molarity. there are two basic types of acid base titrations, indicator and potentiometric. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and. Lab 6 Acid Base Titration.
From www.studypool.com
SOLUTION Lab 6 acid base titrations Studypool Lab 6 Acid Base Titration It is based on the neutralization reaction, where an acid and a base react to form water and a salt. titration is an analytical quantitative technique used to determine the concentration of a solute; By thomas cahill, arizona state university, new college of interdisciplinary arts and sciences. there are two basic types of acid base titrations, indicator and. Lab 6 Acid Base Titration.
From mungfali.com
Acid Base Titration Lab Lab 6 Acid Base Titration study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. The reagent (titrant) is the solution with a known molarity that will react with the analyte. By thomas cahill, arizona state university, new college of interdisciplinary arts and sciences. The analyte (titrand) is the solution with an unknown molarity. use. Lab 6 Acid Base Titration.
From dxosvuizs.blob.core.windows.net
AcidBase Titration Lab Report Sample at Ronald McWilliams blog Lab 6 Acid Base Titration study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. The reagent (titrant) is the solution with a known molarity that will react with the analyte. By thomas cahill, arizona state university, new college of interdisciplinary arts and sciences. titration is an analytical quantitative technique used to determine the concentration. Lab 6 Acid Base Titration.
From www.chegg.com
Solved Experiment 6. AcidBase Titration Name Person Lab 6 Acid Base Titration there are two basic types of acid base titrations, indicator and potentiometric. use the sodium hydroxide, naoh, solution that you standardized in lab 6 as your base of known concentration. The reagent (titrant) is the solution with a known molarity that will react with the analyte. By thomas cahill, arizona state university, new college of interdisciplinary arts and. Lab 6 Acid Base Titration.
From www.studypool.com
SOLUTION Lab Experiment 6 Acid Base Titration Studypool Lab 6 Acid Base Titration titration is an analytical quantitative technique used to determine the concentration of a solute; there are two basic types of acid base titrations, indicator and potentiometric. The reagent (titrant) is the solution with a known molarity that will react with the analyte. It is based on the neutralization reaction, where an acid and a base react to form. Lab 6 Acid Base Titration.
From mungfali.com
Acid Base Titration Lab Lab 6 Acid Base Titration titration is an analytical quantitative technique used to determine the concentration of a solute; use the sodium hydroxide, naoh, solution that you standardized in lab 6 as your base of known concentration. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. there are two basic types. Lab 6 Acid Base Titration.
From mungfali.com
Acid Base Titration Lab Lab 6 Acid Base Titration By thomas cahill, arizona state university, new college of interdisciplinary arts and sciences. there are two basic types of acid base titrations, indicator and potentiometric. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. The analyte (titrand) is the solution with an unknown molarity. use the sodium hydroxide,. Lab 6 Acid Base Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Lab 6 Acid Base Titration The reagent (titrant) is the solution with a known molarity that will react with the analyte. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. there are two basic types of acid base titrations, indicator and potentiometric. By thomas cahill, arizona state university, new college of interdisciplinary arts and. Lab 6 Acid Base Titration.
From mungfali.com
Acid Base Titration Experiment Lab 6 Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. use the sodium hydroxide, naoh, solution that you standardized in lab 6 as your base of known concentration. there are two basic types of acid base titrations, indicator and potentiometric. study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and. Lab 6 Acid Base Titration.