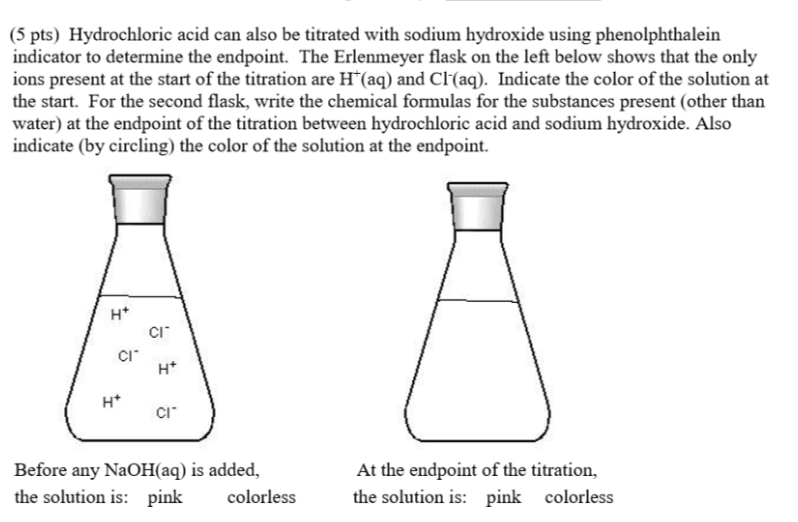
Titration Indicator For Sodium Hydroxide . the reagents are: A typical titration setup is illustrated in the figure below. 2 naoh (s) + co2 (g) →na2co3 (aq) + h2o (l) this means that solid reagent grade sodium hydroxide is. examples of choosing an appropriate indicator for titrations. f a sodium hydroxide (naoh) solution. Titration is a technique used to determine the concentration (molarity) of an unknown solution by allowing it to reac. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. An acid (hydrochloric, sulfuric or nitric) of unknown concentration. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble.
from dxopcuxtp.blob.core.windows.net
the reagents are: A typical titration setup is illustrated in the figure below. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. f a sodium hydroxide (naoh) solution. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble. 2 naoh (s) + co2 (g) →na2co3 (aq) + h2o (l) this means that solid reagent grade sodium hydroxide is. An acid (hydrochloric, sulfuric or nitric) of unknown concentration. examples of choosing an appropriate indicator for titrations. Titration is a technique used to determine the concentration (molarity) of an unknown solution by allowing it to reac.
Titration Experiment Hydrochloric Acid And Sodium Hydroxide at Silvia
Titration Indicator For Sodium Hydroxide the reagents are: Titration is a technique used to determine the concentration (molarity) of an unknown solution by allowing it to reac. 2 naoh (s) + co2 (g) →na2co3 (aq) + h2o (l) this means that solid reagent grade sodium hydroxide is. A typical titration setup is illustrated in the figure below. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. the reagents are: f a sodium hydroxide (naoh) solution. examples of choosing an appropriate indicator for titrations. An acid (hydrochloric, sulfuric or nitric) of unknown concentration. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble.
From www.lazada.co.th
Sodium hydroxide standard solution for titration analysis Lazada.co.th Titration Indicator For Sodium Hydroxide 2 naoh (s) + co2 (g) →na2co3 (aq) + h2o (l) this means that solid reagent grade sodium hydroxide is. A typical titration setup is illustrated in the figure below. Titration is a technique used to determine the concentration (molarity) of an unknown solution by allowing it to reac. the reagents are: An acid (hydrochloric, sulfuric or nitric) of. Titration Indicator For Sodium Hydroxide.
From www.studypool.com
SOLUTION Determination of concentration of sodium hydroxide by Titration Indicator For Sodium Hydroxide one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. f a sodium hydroxide (naoh) solution. the reagents are: An acid (hydrochloric, sulfuric or nitric) of unknown concentration. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble. 2 naoh. Titration Indicator For Sodium Hydroxide.
From www.alamy.com
microscale titration using a plastic pipette and a small vial. Mixing Titration Indicator For Sodium Hydroxide A typical titration setup is illustrated in the figure below. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. 2 naoh (s) + co2 (g) →na2co3 (aq) + h2o (l) this means that solid reagent grade sodium hydroxide is. f a sodium hydroxide (naoh) solution.. Titration Indicator For Sodium Hydroxide.
From studylib.net
Titration of Hydrochloric Acid with Sodium Hydroxide Titration Indicator For Sodium Hydroxide A typical titration setup is illustrated in the figure below. f a sodium hydroxide (naoh) solution. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble. 2 naoh (s) + co2 (g) →na2co3 (aq) + h2o (l) this means that solid reagent grade sodium hydroxide is. the reagents are: examples of choosing. Titration Indicator For Sodium Hydroxide.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Indicator For Sodium Hydroxide f a sodium hydroxide (naoh) solution. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble. Titration is a technique used to determine the concentration (molarity) of an unknown solution by allowing it to reac. one type of titration uses a neutralization reaction, in which an acid and a base react to produce. Titration Indicator For Sodium Hydroxide.
From www.thinkswap.com
Titration of Sodium Hydroxide with Hydrochloric acid FSC107 General Titration Indicator For Sodium Hydroxide 2 naoh (s) + co2 (g) →na2co3 (aq) + h2o (l) this means that solid reagent grade sodium hydroxide is. the reagents are: examples of choosing an appropriate indicator for titrations. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. f a sodium. Titration Indicator For Sodium Hydroxide.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Titration Indicator For Sodium Hydroxide the reagents are: examples of choosing an appropriate indicator for titrations. A typical titration setup is illustrated in the figure below. f a sodium hydroxide (naoh) solution. Titration is a technique used to determine the concentration (molarity) of an unknown solution by allowing it to reac. one type of titration uses a neutralization reaction, in which. Titration Indicator For Sodium Hydroxide.
From www.studypool.com
SOLUTION Volumetric analysis simple titration estimation of sodium Titration Indicator For Sodium Hydroxide examples of choosing an appropriate indicator for titrations. Titration is a technique used to determine the concentration (molarity) of an unknown solution by allowing it to reac. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. A typical titration setup is illustrated in the figure. Titration Indicator For Sodium Hydroxide.
From www.youtube.com
Animation Titration Preparation and Standardization of 1M Sodium Titration Indicator For Sodium Hydroxide the reagents are: 2 naoh (s) + co2 (g) →na2co3 (aq) + h2o (l) this means that solid reagent grade sodium hydroxide is. examples of choosing an appropriate indicator for titrations. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. A typical titration setup. Titration Indicator For Sodium Hydroxide.
From www.numerade.com
SOLVEDmEztion CUNVELAFnment; 1) Predict the titration curve for the Titration Indicator For Sodium Hydroxide examples of choosing an appropriate indicator for titrations. An acid (hydrochloric, sulfuric or nitric) of unknown concentration. A typical titration setup is illustrated in the figure below. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble. the reagents are: Titration is a technique used to determine the concentration (molarity) of an unknown. Titration Indicator For Sodium Hydroxide.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC Titration Indicator For Sodium Hydroxide f a sodium hydroxide (naoh) solution. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. An acid (hydrochloric, sulfuric or nitric) of unknown concentration. A typical titration setup is illustrated in the figure below. in this experiment students neutralise sodium hydroxide with hydrochloric acid. Titration Indicator For Sodium Hydroxide.
From www.youtube.com
Tutorial Titration of Hydrochloric Acid and Sodium Hydroxide YouTube Titration Indicator For Sodium Hydroxide f a sodium hydroxide (naoh) solution. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble. 2 naoh (s) + co2 (g) →na2co3 (aq) + h2o (l) this means that solid reagent grade sodium hydroxide is. Titration is a technique used to determine the concentration (molarity) of an unknown solution by allowing it to. Titration Indicator For Sodium Hydroxide.
From chempedia.info
Sodium hydroxide titration with acetic acid Big Chemical Encyclopedia Titration Indicator For Sodium Hydroxide one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. examples of choosing an appropriate indicator for titrations. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble. Titration is a technique used to determine the concentration (molarity) of an unknown. Titration Indicator For Sodium Hydroxide.
From www.chegg.com
TitrationStandardization of Sodium Hydroxide Titration Indicator For Sodium Hydroxide one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. 2 naoh (s) + co2 (g) →na2co3 (aq) + h2o (l) this means that solid reagent grade sodium hydroxide is. f a sodium hydroxide (naoh) solution. the reagents are: Titration is a technique used to. Titration Indicator For Sodium Hydroxide.
From www.chegg.com
Solved Standardization of Sodium hydroxide Attach graph of Titration Indicator For Sodium Hydroxide examples of choosing an appropriate indicator for titrations. 2 naoh (s) + co2 (g) →na2co3 (aq) + h2o (l) this means that solid reagent grade sodium hydroxide is. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. the reagents are: A typical titration setup. Titration Indicator For Sodium Hydroxide.
From www.stuvia.com
Titration of Sodium Hydroxide with HCI (using an indicator) and Titration Indicator For Sodium Hydroxide Titration is a technique used to determine the concentration (molarity) of an unknown solution by allowing it to reac. A typical titration setup is illustrated in the figure below. 2 naoh (s) + co2 (g) →na2co3 (aq) + h2o (l) this means that solid reagent grade sodium hydroxide is. An acid (hydrochloric, sulfuric or nitric) of unknown concentration. f. Titration Indicator For Sodium Hydroxide.
From dxopcuxtp.blob.core.windows.net
Titration Experiment Hydrochloric Acid And Sodium Hydroxide at Silvia Titration Indicator For Sodium Hydroxide A typical titration setup is illustrated in the figure below. 2 naoh (s) + co2 (g) →na2co3 (aq) + h2o (l) this means that solid reagent grade sodium hydroxide is. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble. An acid (hydrochloric, sulfuric or nitric) of unknown concentration. one type of titration uses. Titration Indicator For Sodium Hydroxide.
From www.scribd.com
Titration of Sodium Hydroxide by Using Oxalic Acid 4 PDF Titration Titration Indicator For Sodium Hydroxide one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble. An acid (hydrochloric, sulfuric or nitric) of unknown concentration. the reagents are: 2 naoh (s) + co2 (g) →na2co3 (aq) +. Titration Indicator For Sodium Hydroxide.
From www.buzzle.com
Titration of Sulfuric Acid and Sodium Hydroxide Titration Indicator For Sodium Hydroxide An acid (hydrochloric, sulfuric or nitric) of unknown concentration. Titration is a technique used to determine the concentration (molarity) of an unknown solution by allowing it to reac. the reagents are: in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble. f a sodium hydroxide (naoh) solution. one type of titration uses. Titration Indicator For Sodium Hydroxide.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID9336460 Titration Indicator For Sodium Hydroxide in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble. examples of choosing an appropriate indicator for titrations. the reagents are: Titration is a technique used to determine the concentration (molarity) of an unknown solution by allowing it to reac. A typical titration setup is illustrated in the figure below. f a. Titration Indicator For Sodium Hydroxide.
From www.alamy.com
microscale titration using a plastic pipette and a small vial. Mixing Titration Indicator For Sodium Hydroxide in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble. 2 naoh (s) + co2 (g) →na2co3 (aq) + h2o (l) this means that solid reagent grade sodium hydroxide is. Titration is a technique used to determine the concentration (molarity) of an unknown solution by allowing it to reac. f a sodium hydroxide (naoh). Titration Indicator For Sodium Hydroxide.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Indicator For Sodium Hydroxide 2 naoh (s) + co2 (g) →na2co3 (aq) + h2o (l) this means that solid reagent grade sodium hydroxide is. An acid (hydrochloric, sulfuric or nitric) of unknown concentration. A typical titration setup is illustrated in the figure below. f a sodium hydroxide (naoh) solution. Titration is a technique used to determine the concentration (molarity) of an unknown solution. Titration Indicator For Sodium Hydroxide.
From dxopcuxtp.blob.core.windows.net
Titration Experiment Hydrochloric Acid And Sodium Hydroxide at Silvia Titration Indicator For Sodium Hydroxide An acid (hydrochloric, sulfuric or nitric) of unknown concentration. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. the reagents are: f a sodium hydroxide (naoh) solution. 2 naoh. Titration Indicator For Sodium Hydroxide.
From www.youtube.com
Standardization of a Sodium Hydroxide Solution with a Titration YouTube Titration Indicator For Sodium Hydroxide in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble. Titration is a technique used to determine the concentration (molarity) of an unknown solution by allowing it to reac. examples of choosing an appropriate indicator for titrations. 2 naoh (s) + co2 (g) →na2co3 (aq) + h2o (l) this means that solid reagent grade. Titration Indicator For Sodium Hydroxide.
From exogrvkja.blob.core.windows.net
Indicators For Titrations at Leslie Jackson blog Titration Indicator For Sodium Hydroxide examples of choosing an appropriate indicator for titrations. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble. Titration is a technique used to determine the concentration (molarity) of an unknown solution by allowing it to reac. the reagents are: A typical titration setup is illustrated in the figure below. 2 naoh (s). Titration Indicator For Sodium Hydroxide.
From studykaki.com
Describe a method of preparing sodium chloride from the reaction of Titration Indicator For Sodium Hydroxide one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. Titration is a technique used to determine the concentration (molarity) of an unknown solution by allowing it to reac. 2 naoh (s) + co2 (g) →na2co3 (aq) + h2o (l) this means that solid reagent grade sodium. Titration Indicator For Sodium Hydroxide.
From www.scribd.com
Standardization of a Base Titration Sodium Hydroxide Titration Indicator For Sodium Hydroxide Titration is a technique used to determine the concentration (molarity) of an unknown solution by allowing it to reac. A typical titration setup is illustrated in the figure below. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. 2 naoh (s) + co2 (g) →na2co3 (aq). Titration Indicator For Sodium Hydroxide.
From exovmncod.blob.core.windows.net
Indicator Definition In A Titration at Maria Little blog Titration Indicator For Sodium Hydroxide in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble. 2 naoh (s) + co2 (g) →na2co3 (aq) + h2o (l) this means that solid reagent grade sodium hydroxide is. f a sodium hydroxide (naoh) solution. Titration is a technique used to determine the concentration (molarity) of an unknown solution by allowing it to. Titration Indicator For Sodium Hydroxide.
From dxodkszcm.blob.core.windows.net
Titration In Chemistry Is at Laura Fraser blog Titration Indicator For Sodium Hydroxide examples of choosing an appropriate indicator for titrations. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. Titration is a technique used to determine the concentration (molarity) of an unknown solution by allowing it to reac. the reagents are: A typical titration setup is. Titration Indicator For Sodium Hydroxide.
From www.numerade.com
SOLVED Clearly draw the titration curve for the reaction of this Titration Indicator For Sodium Hydroxide A typical titration setup is illustrated in the figure below. Titration is a technique used to determine the concentration (molarity) of an unknown solution by allowing it to reac. the reagents are: f a sodium hydroxide (naoh) solution. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble. 2 naoh (s) + co2. Titration Indicator For Sodium Hydroxide.
From www.tutormyself.com
233 Archives TutorMyself Chemistry Titration Indicator For Sodium Hydroxide Titration is a technique used to determine the concentration (molarity) of an unknown solution by allowing it to reac. A typical titration setup is illustrated in the figure below. examples of choosing an appropriate indicator for titrations. the reagents are: 2 naoh (s) + co2 (g) →na2co3 (aq) + h2o (l) this means that solid reagent grade sodium. Titration Indicator For Sodium Hydroxide.
From www.scribd.com
Cce48Titration of Sodium Hydroxide With Hydrochloric Acid Sodium Titration Indicator For Sodium Hydroxide A typical titration setup is illustrated in the figure below. f a sodium hydroxide (naoh) solution. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. examples of choosing an appropriate indicator for titrations. An acid (hydrochloric, sulfuric or nitric) of unknown concentration. the. Titration Indicator For Sodium Hydroxide.
From www.scribd.com
BCB 103L Expt 6 Titration of Sodium Hydroxide With Oxalic Acid Using Titration Indicator For Sodium Hydroxide one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. the reagents are: A typical titration setup is illustrated in the figure below. examples of choosing an appropriate indicator for titrations. Titration is a technique used to determine the concentration (molarity) of an unknown solution. Titration Indicator For Sodium Hydroxide.
From www.chegg.com
Solved TITRATION ⋅ STANDARDIZATION OF SODIUM HYDROXIDE Titration Indicator For Sodium Hydroxide f a sodium hydroxide (naoh) solution. 2 naoh (s) + co2 (g) →na2co3 (aq) + h2o (l) this means that solid reagent grade sodium hydroxide is. one type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water. in this experiment students neutralise sodium hydroxide with hydrochloric. Titration Indicator For Sodium Hydroxide.
From www.chegg.com
Solved TITRATION STANDARDIZATION OF SODIUM HYDROXIDE SUBMIT Titration Indicator For Sodium Hydroxide the reagents are: An acid (hydrochloric, sulfuric or nitric) of unknown concentration. A typical titration setup is illustrated in the figure below. f a sodium hydroxide (naoh) solution. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble. 2 naoh (s) + co2 (g) →na2co3 (aq) + h2o (l) this means that solid. Titration Indicator For Sodium Hydroxide.