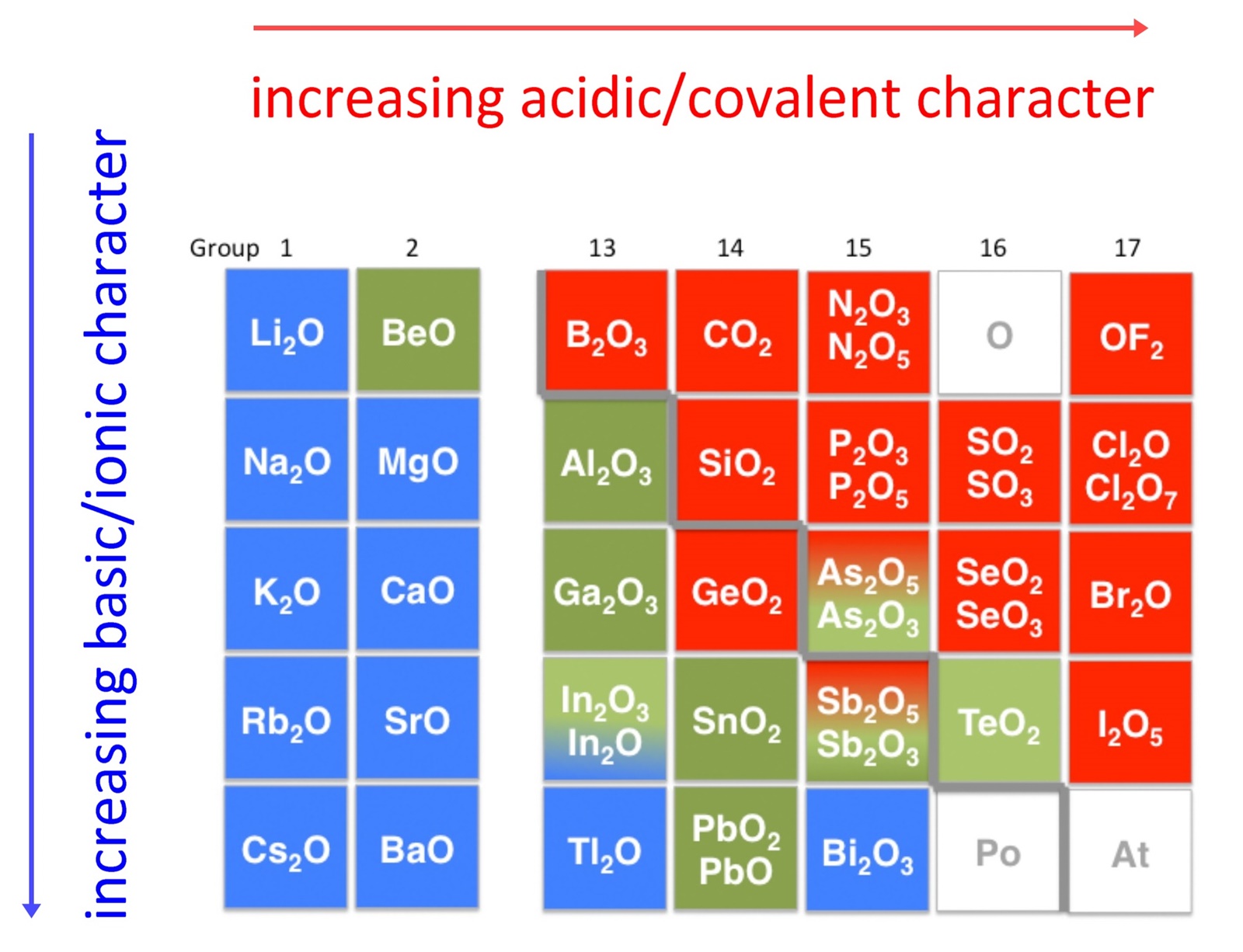
The Element X Of Atomic Number 16 Forms Basic Oxide . Metals generally form basic oxides. In general, the electropositive character of the oxide's central atom will determine whether the oxide will be acidic or basic. The more electropositive the central. (a) 7 (2,5) (b) 17 (2,8,7) (c) 14 (2,8,4) (d) 11 (2,8,1) answer: An element with atomic number___ will form a basic oxide. Some elements in the center of the periodic table form amphoteric oxides, which will react with acids and. The element 'x' of atomic number 16 forms oxide. An element with atomic number 11 (2, 8, 1) will form a basic oxide. (d) 11 (2,8,1) basic oxides are formed by the. Nonmetals generally form acidic oxides. Basic oxides are oxides that show basic properties, in opposition to acidic oxides. As the atomic number of the element is 16 means. Metals form basic oxides with 1 to 3 electrons in their. A basic oxide can either react with water to form a.
from chem.libretexts.org
The element 'x' of atomic number 16 forms oxide. Metals generally form basic oxides. (d) 11 (2,8,1) basic oxides are formed by the. Nonmetals generally form acidic oxides. Some elements in the center of the periodic table form amphoteric oxides, which will react with acids and. Metals form basic oxides with 1 to 3 electrons in their. An element with atomic number___ will form a basic oxide. The more electropositive the central. Basic oxides are oxides that show basic properties, in opposition to acidic oxides. A basic oxide can either react with water to form a.
7.8A Amphoteric Behavior Chemistry LibreTexts
The Element X Of Atomic Number 16 Forms Basic Oxide (a) 7 (2,5) (b) 17 (2,8,7) (c) 14 (2,8,4) (d) 11 (2,8,1) answer: In general, the electropositive character of the oxide's central atom will determine whether the oxide will be acidic or basic. (d) 11 (2,8,1) basic oxides are formed by the. A basic oxide can either react with water to form a. Nonmetals generally form acidic oxides. The element 'x' of atomic number 16 forms oxide. As the atomic number of the element is 16 means. Basic oxides are oxides that show basic properties, in opposition to acidic oxides. An element with atomic number 11 (2, 8, 1) will form a basic oxide. Metals form basic oxides with 1 to 3 electrons in their. (a) 7 (2,5) (b) 17 (2,8,7) (c) 14 (2,8,4) (d) 11 (2,8,1) answer: An element with atomic number___ will form a basic oxide. The more electropositive the central. Metals generally form basic oxides. Some elements in the center of the periodic table form amphoteric oxides, which will react with acids and.
From www.oceanproperty.co.th
Periodic Table Of Elements With Names And Symbols And Atomic Mass And The Element X Of Atomic Number 16 Forms Basic Oxide Metals generally form basic oxides. (a) 7 (2,5) (b) 17 (2,8,7) (c) 14 (2,8,4) (d) 11 (2,8,1) answer: The element 'x' of atomic number 16 forms oxide. An element with atomic number___ will form a basic oxide. Basic oxides are oxides that show basic properties, in opposition to acidic oxides. (d) 11 (2,8,1) basic oxides are formed by the. Metals. The Element X Of Atomic Number 16 Forms Basic Oxide.
From periodictable.me
Atomic Number Examples Archives Dynamic Periodic Table of Elements The Element X Of Atomic Number 16 Forms Basic Oxide The element 'x' of atomic number 16 forms oxide. In general, the electropositive character of the oxide's central atom will determine whether the oxide will be acidic or basic. An element with atomic number___ will form a basic oxide. Basic oxides are oxides that show basic properties, in opposition to acidic oxides. Nonmetals generally form acidic oxides. (a) 7 (2,5). The Element X Of Atomic Number 16 Forms Basic Oxide.
From courses.lumenlearning.com
The Periodic Table of Elements Biology for Majors I The Element X Of Atomic Number 16 Forms Basic Oxide The more electropositive the central. Basic oxides are oxides that show basic properties, in opposition to acidic oxides. The element 'x' of atomic number 16 forms oxide. Metals form basic oxides with 1 to 3 electrons in their. (a) 7 (2,5) (b) 17 (2,8,7) (c) 14 (2,8,4) (d) 11 (2,8,1) answer: Nonmetals generally form acidic oxides. Some elements in the. The Element X Of Atomic Number 16 Forms Basic Oxide.
From www.gkseries.com
Which of the following is the atomic number of an element that forms The Element X Of Atomic Number 16 Forms Basic Oxide Metals form basic oxides with 1 to 3 electrons in their. A basic oxide can either react with water to form a. In general, the electropositive character of the oxide's central atom will determine whether the oxide will be acidic or basic. (a) 7 (2,5) (b) 17 (2,8,7) (c) 14 (2,8,4) (d) 11 (2,8,1) answer: Basic oxides are oxides that. The Element X Of Atomic Number 16 Forms Basic Oxide.
From www.sciencephoto.com
Oxygen, atomic structure Stock Image C018/3689 Science Photo Library The Element X Of Atomic Number 16 Forms Basic Oxide (a) 7 (2,5) (b) 17 (2,8,7) (c) 14 (2,8,4) (d) 11 (2,8,1) answer: An element with atomic number 11 (2, 8, 1) will form a basic oxide. The more electropositive the central. Nonmetals generally form acidic oxides. In general, the electropositive character of the oxide's central atom will determine whether the oxide will be acidic or basic. Some elements in. The Element X Of Atomic Number 16 Forms Basic Oxide.
From courses.lumenlearning.com
The Periodic Table Chemistry for Majors The Element X Of Atomic Number 16 Forms Basic Oxide Basic oxides are oxides that show basic properties, in opposition to acidic oxides. As the atomic number of the element is 16 means. (a) 7 (2,5) (b) 17 (2,8,7) (c) 14 (2,8,4) (d) 11 (2,8,1) answer: The element 'x' of atomic number 16 forms oxide. Some elements in the center of the periodic table form amphoteric oxides, which will react. The Element X Of Atomic Number 16 Forms Basic Oxide.
From periodictable.me
Printable Periodic table with atomic number Dynamic Periodic Table of The Element X Of Atomic Number 16 Forms Basic Oxide Metals generally form basic oxides. (a) 7 (2,5) (b) 17 (2,8,7) (c) 14 (2,8,4) (d) 11 (2,8,1) answer: An element with atomic number 11 (2, 8, 1) will form a basic oxide. Metals form basic oxides with 1 to 3 electrons in their. The element 'x' of atomic number 16 forms oxide. Nonmetals generally form acidic oxides. A basic oxide. The Element X Of Atomic Number 16 Forms Basic Oxide.
From elchoroukhost.net
Periodic Table With Oxidation Numbers And Names Elcho Table The Element X Of Atomic Number 16 Forms Basic Oxide As the atomic number of the element is 16 means. The more electropositive the central. A basic oxide can either react with water to form a. (d) 11 (2,8,1) basic oxides are formed by the. An element with atomic number___ will form a basic oxide. Nonmetals generally form acidic oxides. Metals form basic oxides with 1 to 3 electrons in. The Element X Of Atomic Number 16 Forms Basic Oxide.
From www.alamy.com
Periodic Table of the Elements shows atomic number, symbol, name The Element X Of Atomic Number 16 Forms Basic Oxide Nonmetals generally form acidic oxides. The more electropositive the central. Metals form basic oxides with 1 to 3 electrons in their. As the atomic number of the element is 16 means. (a) 7 (2,5) (b) 17 (2,8,7) (c) 14 (2,8,4) (d) 11 (2,8,1) answer: Some elements in the center of the periodic table form amphoteric oxides, which will react with. The Element X Of Atomic Number 16 Forms Basic Oxide.
From periodictable.me
Modern Periodic Table of Elements with Names and Symbols The Element X Of Atomic Number 16 Forms Basic Oxide Basic oxides are oxides that show basic properties, in opposition to acidic oxides. Nonmetals generally form acidic oxides. As the atomic number of the element is 16 means. The element 'x' of atomic number 16 forms oxide. In general, the electropositive character of the oxide's central atom will determine whether the oxide will be acidic or basic. Metals form basic. The Element X Of Atomic Number 16 Forms Basic Oxide.
From animalia-life.club
Element Symbols Periodic Table The Element X Of Atomic Number 16 Forms Basic Oxide The more electropositive the central. (a) 7 (2,5) (b) 17 (2,8,7) (c) 14 (2,8,4) (d) 11 (2,8,1) answer: The element 'x' of atomic number 16 forms oxide. Metals form basic oxides with 1 to 3 electrons in their. In general, the electropositive character of the oxide's central atom will determine whether the oxide will be acidic or basic. Some elements. The Element X Of Atomic Number 16 Forms Basic Oxide.
From brokeasshome.com
Printable Periodic Table With Names Of Elements And Atomic Numbers The Element X Of Atomic Number 16 Forms Basic Oxide Metals generally form basic oxides. An element with atomic number___ will form a basic oxide. The element 'x' of atomic number 16 forms oxide. Nonmetals generally form acidic oxides. Some elements in the center of the periodic table form amphoteric oxides, which will react with acids and. (d) 11 (2,8,1) basic oxides are formed by the. A basic oxide can. The Element X Of Atomic Number 16 Forms Basic Oxide.
From mavink.com
Periodic Table Simplified The Element X Of Atomic Number 16 Forms Basic Oxide The more electropositive the central. (d) 11 (2,8,1) basic oxides are formed by the. As the atomic number of the element is 16 means. An element with atomic number___ will form a basic oxide. Some elements in the center of the periodic table form amphoteric oxides, which will react with acids and. In general, the electropositive character of the oxide's. The Element X Of Atomic Number 16 Forms Basic Oxide.
From periodictable.me
Printable Periodic table with atomic number Dynamic Periodic Table of The Element X Of Atomic Number 16 Forms Basic Oxide Metals form basic oxides with 1 to 3 electrons in their. In general, the electropositive character of the oxide's central atom will determine whether the oxide will be acidic or basic. (a) 7 (2,5) (b) 17 (2,8,7) (c) 14 (2,8,4) (d) 11 (2,8,1) answer: As the atomic number of the element is 16 means. The more electropositive the central. Metals. The Element X Of Atomic Number 16 Forms Basic Oxide.
From dxocoycqq.blob.core.windows.net
What Element Has A Atomic Number Of 16 at Delphia Atkins blog The Element X Of Atomic Number 16 Forms Basic Oxide A basic oxide can either react with water to form a. (d) 11 (2,8,1) basic oxides are formed by the. In general, the electropositive character of the oxide's central atom will determine whether the oxide will be acidic or basic. As the atomic number of the element is 16 means. Nonmetals generally form acidic oxides. Metals form basic oxides with. The Element X Of Atomic Number 16 Forms Basic Oxide.
From www.thoughtco.com
Element List Atomic Number, Element Name and Symbol The Element X Of Atomic Number 16 Forms Basic Oxide Metals generally form basic oxides. The element 'x' of atomic number 16 forms oxide. An element with atomic number 11 (2, 8, 1) will form a basic oxide. Nonmetals generally form acidic oxides. Some elements in the center of the periodic table form amphoteric oxides, which will react with acids and. Basic oxides are oxides that show basic properties, in. The Element X Of Atomic Number 16 Forms Basic Oxide.
From www.britannica.com
periodic table Definition, Elements, Groups, Charges, Trends, & Facts The Element X Of Atomic Number 16 Forms Basic Oxide The element 'x' of atomic number 16 forms oxide. An element with atomic number 11 (2, 8, 1) will form a basic oxide. Metals generally form basic oxides. (d) 11 (2,8,1) basic oxides are formed by the. As the atomic number of the element is 16 means. Some elements in the center of the periodic table form amphoteric oxides, which. The Element X Of Atomic Number 16 Forms Basic Oxide.
From utedzz.blogspot.com
Periodic Table Atomic Number 16 Periodic Table Timeline The Element X Of Atomic Number 16 Forms Basic Oxide A basic oxide can either react with water to form a. Nonmetals generally form acidic oxides. (a) 7 (2,5) (b) 17 (2,8,7) (c) 14 (2,8,4) (d) 11 (2,8,1) answer: Metals form basic oxides with 1 to 3 electrons in their. The element 'x' of atomic number 16 forms oxide. Some elements in the center of the periodic table form amphoteric. The Element X Of Atomic Number 16 Forms Basic Oxide.
From chem.libretexts.org
7.8A Amphoteric Behavior Chemistry LibreTexts The Element X Of Atomic Number 16 Forms Basic Oxide (d) 11 (2,8,1) basic oxides are formed by the. Nonmetals generally form acidic oxides. Metals generally form basic oxides. The more electropositive the central. The element 'x' of atomic number 16 forms oxide. Metals form basic oxides with 1 to 3 electrons in their. In general, the electropositive character of the oxide's central atom will determine whether the oxide will. The Element X Of Atomic Number 16 Forms Basic Oxide.
From www.priyamstudycentre.com
Periodic Table Elements, Definition, Groups, Periods, Blocks The Element X Of Atomic Number 16 Forms Basic Oxide The element 'x' of atomic number 16 forms oxide. (d) 11 (2,8,1) basic oxides are formed by the. In general, the electropositive character of the oxide's central atom will determine whether the oxide will be acidic or basic. (a) 7 (2,5) (b) 17 (2,8,7) (c) 14 (2,8,4) (d) 11 (2,8,1) answer: The more electropositive the central. As the atomic number. The Element X Of Atomic Number 16 Forms Basic Oxide.
From alevelchemistry.co.uk
Electron Structure ALevel Chemistry Revision Notes The Element X Of Atomic Number 16 Forms Basic Oxide (d) 11 (2,8,1) basic oxides are formed by the. Nonmetals generally form acidic oxides. An element with atomic number 11 (2, 8, 1) will form a basic oxide. A basic oxide can either react with water to form a. In general, the electropositive character of the oxide's central atom will determine whether the oxide will be acidic or basic. Metals. The Element X Of Atomic Number 16 Forms Basic Oxide.
From mungfali.com
Printable Periodic Table With Names Of Elements And Atomic Numbers 5C0 The Element X Of Atomic Number 16 Forms Basic Oxide In general, the electropositive character of the oxide's central atom will determine whether the oxide will be acidic or basic. Basic oxides are oxides that show basic properties, in opposition to acidic oxides. A basic oxide can either react with water to form a. The more electropositive the central. As the atomic number of the element is 16 means. Metals. The Element X Of Atomic Number 16 Forms Basic Oxide.
From freehomedelivery.net
Periodic Table Periodic Table Of Elements, Table Of Elements, Modern The Element X Of Atomic Number 16 Forms Basic Oxide (d) 11 (2,8,1) basic oxides are formed by the. (a) 7 (2,5) (b) 17 (2,8,7) (c) 14 (2,8,4) (d) 11 (2,8,1) answer: A basic oxide can either react with water to form a. The element 'x' of atomic number 16 forms oxide. An element with atomic number___ will form a basic oxide. Metals generally form basic oxides. Nonmetals generally form. The Element X Of Atomic Number 16 Forms Basic Oxide.
From commons.wikimedia.org
FilePeriodic table of elements showing electron shells.png The Element X Of Atomic Number 16 Forms Basic Oxide The element 'x' of atomic number 16 forms oxide. The more electropositive the central. As the atomic number of the element is 16 means. Basic oxides are oxides that show basic properties, in opposition to acidic oxides. Nonmetals generally form acidic oxides. (d) 11 (2,8,1) basic oxides are formed by the. A basic oxide can either react with water to. The Element X Of Atomic Number 16 Forms Basic Oxide.
From dxocoycqq.blob.core.windows.net
What Element Has A Atomic Number Of 16 at Delphia Atkins blog The Element X Of Atomic Number 16 Forms Basic Oxide An element with atomic number 11 (2, 8, 1) will form a basic oxide. Metals form basic oxides with 1 to 3 electrons in their. A basic oxide can either react with water to form a. As the atomic number of the element is 16 means. (a) 7 (2,5) (b) 17 (2,8,7) (c) 14 (2,8,4) (d) 11 (2,8,1) answer: Metals. The Element X Of Atomic Number 16 Forms Basic Oxide.
From utedzz.blogspot.com
Periodic Table With Names Of Elements And Symbols Periodic Table Timeline The Element X Of Atomic Number 16 Forms Basic Oxide Basic oxides are oxides that show basic properties, in opposition to acidic oxides. In general, the electropositive character of the oxide's central atom will determine whether the oxide will be acidic or basic. An element with atomic number 11 (2, 8, 1) will form a basic oxide. Metals form basic oxides with 1 to 3 electrons in their. Nonmetals generally. The Element X Of Atomic Number 16 Forms Basic Oxide.
From www.tpsearchtool.com
Periodic Table Of Elements With Names And Symbols And Atomic Mass And The Element X Of Atomic Number 16 Forms Basic Oxide Metals generally form basic oxides. Basic oxides are oxides that show basic properties, in opposition to acidic oxides. (d) 11 (2,8,1) basic oxides are formed by the. Some elements in the center of the periodic table form amphoteric oxides, which will react with acids and. An element with atomic number 11 (2, 8, 1) will form a basic oxide. In. The Element X Of Atomic Number 16 Forms Basic Oxide.
From elchoroukhost.net
Periodic Table With Oxidation Numbers And Names Elcho Table The Element X Of Atomic Number 16 Forms Basic Oxide (a) 7 (2,5) (b) 17 (2,8,7) (c) 14 (2,8,4) (d) 11 (2,8,1) answer: An element with atomic number 11 (2, 8, 1) will form a basic oxide. Nonmetals generally form acidic oxides. Basic oxides are oxides that show basic properties, in opposition to acidic oxides. The more electropositive the central. The element 'x' of atomic number 16 forms oxide. A. The Element X Of Atomic Number 16 Forms Basic Oxide.
From awesomehome.co
Periodic Table Of Elements List With Protons Neutrons And Electrons The Element X Of Atomic Number 16 Forms Basic Oxide Metals generally form basic oxides. Metals form basic oxides with 1 to 3 electrons in their. Some elements in the center of the periodic table form amphoteric oxides, which will react with acids and. As the atomic number of the element is 16 means. An element with atomic number 11 (2, 8, 1) will form a basic oxide. (d) 11. The Element X Of Atomic Number 16 Forms Basic Oxide.
From utedzz.blogspot.com
Periodic Table Atomic Number 16 Periodic Table Timeline The Element X Of Atomic Number 16 Forms Basic Oxide An element with atomic number 11 (2, 8, 1) will form a basic oxide. A basic oxide can either react with water to form a. In general, the electropositive character of the oxide's central atom will determine whether the oxide will be acidic or basic. Nonmetals generally form acidic oxides. Metals form basic oxides with 1 to 3 electrons in. The Element X Of Atomic Number 16 Forms Basic Oxide.
From chemassist.blogspot.com
ChemAssist Elements and the Periodic Table The Element X Of Atomic Number 16 Forms Basic Oxide An element with atomic number 11 (2, 8, 1) will form a basic oxide. (d) 11 (2,8,1) basic oxides are formed by the. Metals generally form basic oxides. Basic oxides are oxides that show basic properties, in opposition to acidic oxides. In general, the electropositive character of the oxide's central atom will determine whether the oxide will be acidic or. The Element X Of Atomic Number 16 Forms Basic Oxide.
From ar.inspiredpencil.com
Periodic Table Of Elements With Atomic Mass The Element X Of Atomic Number 16 Forms Basic Oxide The element 'x' of atomic number 16 forms oxide. An element with atomic number 11 (2, 8, 1) will form a basic oxide. (a) 7 (2,5) (b) 17 (2,8,7) (c) 14 (2,8,4) (d) 11 (2,8,1) answer: Nonmetals generally form acidic oxides. The more electropositive the central. An element with atomic number___ will form a basic oxide. (d) 11 (2,8,1) basic. The Element X Of Atomic Number 16 Forms Basic Oxide.
From iperiodictable.com
Printable Periodic Table With Names, Atomic Mass or Number The Element X Of Atomic Number 16 Forms Basic Oxide The element 'x' of atomic number 16 forms oxide. The more electropositive the central. Metals form basic oxides with 1 to 3 electrons in their. A basic oxide can either react with water to form a. An element with atomic number 11 (2, 8, 1) will form a basic oxide. An element with atomic number___ will form a basic oxide.. The Element X Of Atomic Number 16 Forms Basic Oxide.
From www.thoughtco.com
Element List Atomic Number, Element Name and Symbol The Element X Of Atomic Number 16 Forms Basic Oxide Metals form basic oxides with 1 to 3 electrons in their. The more electropositive the central. An element with atomic number 11 (2, 8, 1) will form a basic oxide. In general, the electropositive character of the oxide's central atom will determine whether the oxide will be acidic or basic. The element 'x' of atomic number 16 forms oxide. Nonmetals. The Element X Of Atomic Number 16 Forms Basic Oxide.
From dxocoycqq.blob.core.windows.net
What Element Has A Atomic Number Of 16 at Delphia Atkins blog The Element X Of Atomic Number 16 Forms Basic Oxide An element with atomic number___ will form a basic oxide. (a) 7 (2,5) (b) 17 (2,8,7) (c) 14 (2,8,4) (d) 11 (2,8,1) answer: A basic oxide can either react with water to form a. Metals generally form basic oxides. Metals form basic oxides with 1 to 3 electrons in their. Some elements in the center of the periodic table form. The Element X Of Atomic Number 16 Forms Basic Oxide.