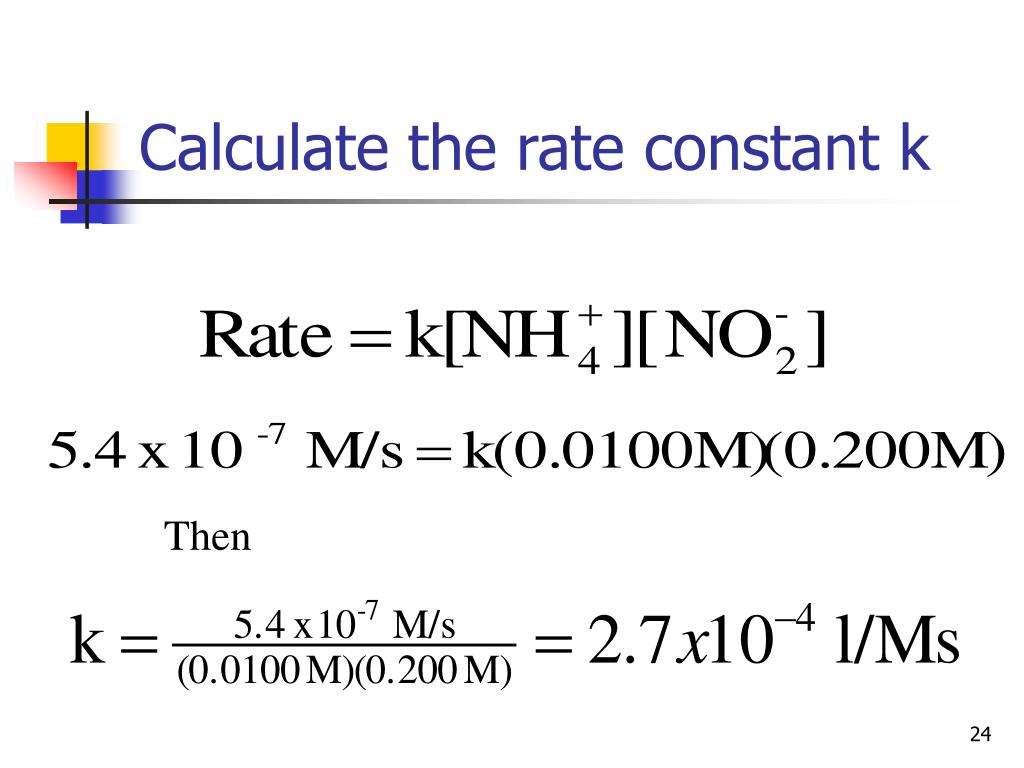
Rate Constant K . It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. See how catalysts can lower the activation energy and increase the rate of reaction. The rateconstant (k) of a reaction can be calculated using: The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. The rate constant k is. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. Learn how rate constants vary with temperature and activation energy according to the arrhenius equation. The initialrates and the rate equation. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant.
from www.slideserve.com
The rateconstant (k) of a reaction can be calculated using: The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. See how catalysts can lower the activation energy and increase the rate of reaction. The initialrates and the rate equation. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. The rate constant k is. Learn how rate constants vary with temperature and activation energy according to the arrhenius equation.
PPT Chapter 12 Chemical PowerPoint Presentation, free
Rate Constant K The rate constant k is. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. Learn how rate constants vary with temperature and activation energy according to the arrhenius equation. The rate constant k is. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. The initialrates and the rate equation. The rateconstant (k) of a reaction can be calculated using: See how catalysts can lower the activation energy and increase the rate of reaction.
From www.doubtnut.com
Plots showing the variation of the rate constant (k) with temperature Rate Constant K The rateconstant (k) of a reaction can be calculated using: The rate constant k is. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. The rate constant, k,. Rate Constant K.
From www.researchgate.net
Firstorder reaction rate constants (k obs ) and initial reaction rates Rate Constant K The rateconstant (k) of a reaction can be calculated using: It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. See how catalysts can lower the activation energy. Rate Constant K.
From www.slideserve.com
PPT Chapter 13 Chemical PowerPoint Presentation, free Rate Constant K It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. See how catalysts can lower the activation energy and increase the rate of reaction. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. The rate constant k. Rate Constant K.
From www.pdfprof.com
arrhenius equation graph Rate Constant K The rate constant k is. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. The initialrates and the rate equation. See how catalysts can lower the activation energy and increase the rate of reaction. The rate constant, k, is a number that connects the concentration of reactants in a reaction. Rate Constant K.
From www.slideshare.net
Chemical Rate Constant K The rate constant k is. The initialrates and the rate equation. Learn how rate constants vary with temperature and activation energy according to the arrhenius equation. See how catalysts can lower the activation energy and increase the rate of reaction. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of. Rate Constant K.
From www.slideserve.com
PPT The Equilibrium Constant, K, and The Reaction Quotient, Q Rate Constant K The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. The initialrates and the rate equation. A rate law is an expression showing. Rate Constant K.
From www.chemistrystudent.com
The Rate Equation (ALevel) ChemistryStudent Rate Constant K The initialrates and the rate equation. See how catalysts can lower the activation energy and increase the rate of reaction. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. The rateconstant (k) of a reaction can be calculated using: The rate constant is a proportionality factor in the rate law. Rate Constant K.
From www.youtube.com
units of the rate constant k derivations YouTube Rate Constant K It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. The initialrates and the rate equation. The rate constant is a proportionality factor in the rate law of chemical. Rate Constant K.
From www.youtube.com
Determine the rate constant (k) for a reaction YouTube Rate Constant K A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. It is also known as the reaction rate constant or reaction rate coefficient and is indicated in. Rate Constant K.
From www.youtube.com
Units of rate constant calculate rate constant units units of first Rate Constant K The rateconstant (k) of a reaction can be calculated using: A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. Learn how rate constants vary with temperature and activation energy according to the arrhenius equation. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates. Rate Constant K.
From www.solvedlib.com
Calculate three values for the rate constant; k, at r… SolvedLib Rate Constant K The initialrates and the rate equation. See how catalysts can lower the activation energy and increase the rate of reaction. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that. Rate Constant K.
From www.slideshare.net
Chapter 14 Lecture Chemical Rate Constant K The rate constant k is. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. Learn how rate constants vary with temperature and activation energy according to the arrhenius equation. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. The rate constant. Rate Constant K.
From www.chegg.com
Solved What are the units of the rate constant (k) for a Rate Constant K It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. Learn how to write and use rate laws to describe the relationship between. Rate Constant K.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID315859 Rate Constant K Learn how rate constants vary with temperature and activation energy according to the arrhenius equation. The rateconstant (k) of a reaction can be calculated using: The rate constant k is. The initialrates and the rate equation. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate.. Rate Constant K.
From www.doubtnut.com
Plots showing the variation of the rate constant (k) with temperature Rate Constant K See how catalysts can lower the activation energy and increase the rate of reaction. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. The initialrates and the rate equation. The rate constant k is. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates. Rate Constant K.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical Rate Constant K See how catalysts can lower the activation energy and increase the rate of reaction. The rate constant k is. The rateconstant (k) of a reaction can be calculated using: The initialrates and the rate equation. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. Learn how rate constants vary with temperature. Rate Constant K.
From www.slideserve.com
PPT Chemistry 102(01) Spring 2012 PowerPoint Presentation, free Rate Constant K It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. The rateconstant (k) of a reaction can be calculated using: The initialrates and the rate equation. See how catalysts can lower the activation energy and increase the rate of reaction. Learn how rate constants vary with temperature. Rate Constant K.
From www.youtube.com
How to Find the Rate Law and Rate Constant (k) YouTube Rate Constant K See how catalysts can lower the activation energy and increase the rate of reaction. The initialrates and the rate equation. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations.. Rate Constant K.
From www.youtube.com
The rate constant `(K\')` of one reaction is double of the rate Rate Constant K The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. Learn how rate constants vary with temperature and activation energy according to the arrhenius equation. The rateconstant (k) of a reaction can be calculated using: See how catalysts can lower the activation energy and increase the. Rate Constant K.
From www.youtube.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc YouTube Rate Constant K Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. Learn how rate constants vary with temperature and activation energy according to the arrhenius equation. The initialrates and the rate equation. The rateconstant (k) of a reaction can be calculated using: A rate law is an expression showing the relationship of the. Rate Constant K.
From www.doubtnut.com
Plots showing the variation of the rate constant (k) with temperature Rate Constant K It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The initialrates and the rate equation. The rate constant is a proportionality factor in the. Rate Constant K.
From pdfprof.com
arrhenius equation r Rate Constant K Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. The rateconstant (k) of a reaction can be calculated using: A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. The rate constant k is. The rate constant is a proportionality factor in. Rate Constant K.
From www.youtube.com
Intro to Rate Laws, Rate Constants, Reaction Order Chemistry Tutorial Rate Constant K The rate constant k is. See how catalysts can lower the activation energy and increase the rate of reaction. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. The rateconstant (k) of a reaction can be calculated using: The rate constant, k, is a number that connects the concentration of reactants. Rate Constant K.
From www.researchgate.net
Arrhenius diagram for the rate constants k 1 and k 2 (s À1 Download Rate Constant K The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. The rate constant k is. See how catalysts can lower the activation energy and increase the rate of reaction. Learn how rate constants vary with temperature and activation energy according to the arrhenius equation. It is. Rate Constant K.
From www.slideserve.com
PPT CHEMICAL PowerPoint Presentation, free download ID Rate Constant K Learn how rate constants vary with temperature and activation energy according to the arrhenius equation. The rateconstant (k) of a reaction can be calculated using: The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. See how catalysts can lower the activation energy and increase the. Rate Constant K.
From study.com
Rate Constant & Rate Law Definition, Differences & Examples Lesson Rate Constant K The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. Learn how rate constants vary with temperature and activation energy according to the arrhenius equation. The initialrates and the rate equation. The rate constant, k, is a number that connects the concentration of reactants in a. Rate Constant K.
From www.sliderbase.com
Rate Laws Presentation Chemistry Rate Constant K Learn how rate constants vary with temperature and activation energy according to the arrhenius equation. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. The rate constant k is. It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter. Rate Constant K.
From www.slideserve.com
PPT CHEMICAL PowerPoint Presentation ID5609537 Rate Constant K It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. The initialrates and the rate equation. Learn how rate constants vary with temperature and activation energy according to the arrhenius equation. See how catalysts can lower the activation energy and increase the rate of reaction. Learn how. Rate Constant K.
From www.youtube.com
Rate equation and the units of the rate constant YouTube Rate Constant K Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. The rate constant k is. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. Learn how rate constants vary with temperature and activation energy according to the. Rate Constant K.
From www.youtube.com
How to Determine Units of Rate Constant k (Shortcut with Examples Rate Constant K The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. The rate constant k is. A rate law is an expression showing the relationship of the reaction rate to the concentrations of each reactant. The rate constant, k, is a number that connects the concentration of. Rate Constant K.
From www.slideserve.com
PPT Rate laws (2) PowerPoint Presentation, free download ID2351800 Rate Constant K It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. See how catalysts can lower the activation energy and increase the rate of reaction. The rateconstant (k) of a reaction can be calculated using: The rate constant, k, is a number that connects the concentration of reactants. Rate Constant K.
From www.slideserve.com
PPT Chapter 12 Chemical PowerPoint Presentation, free Rate Constant K The initialrates and the rate equation. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. Learn how to write and use rate laws to describe the relationship between reaction rate and reactant concentrations. The rate constant k is. A rate law is an expression showing. Rate Constant K.
From www.sliderbase.com
Rate Laws Presentation Chemistry Rate Constant K The rate constant k is. It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the letter k. Learn how rate constants vary with temperature and activation energy according to the arrhenius equation. The rate constant, k, is a number that connects the concentration of reactants in a reaction to. Rate Constant K.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID6810274 Rate Constant K The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. Learn how rate constants vary with temperature and activation energy according to the arrhenius. Rate Constant K.
From www.slideserve.com
PPT Rate Laws PowerPoint Presentation, free download ID4905523 Rate Constant K Learn how rate constants vary with temperature and activation energy according to the arrhenius equation. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. It is also known as the reaction rate constant or reaction rate coefficient and is indicated in an equation by the. Rate Constant K.