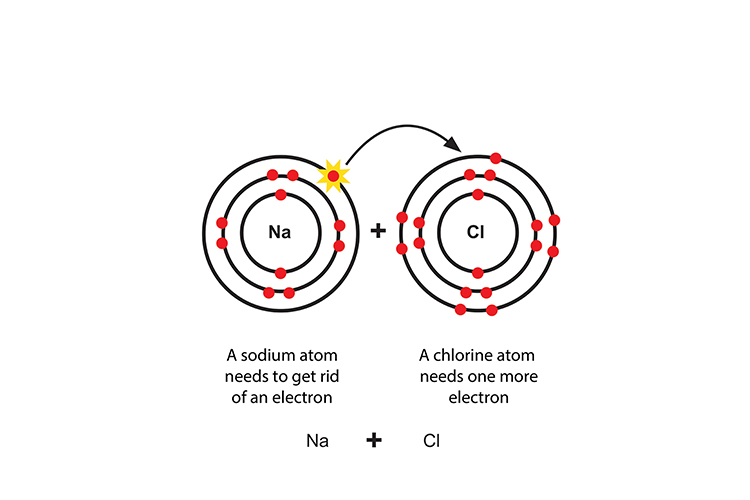
Chlorine Ionic Bond Form . Such a bond forms when the. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine atoms, forming sodium chloride (nacl). The arrow indicates the transfer of the electron from sodium to.
from mammothmemory.net
chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. The arrow indicates the transfer of the electron from sodium to. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine atoms, forming sodium chloride (nacl). Such a bond forms when the.
Chemical bonding is about atoms achieving full outer shells
Chlorine Ionic Bond Form The arrow indicates the transfer of the electron from sodium to. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. The arrow indicates the transfer of the electron from sodium to. Such a bond forms when the. the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine atoms, forming sodium chloride (nacl).
From quizlet.com
5 ) Ionic Bonding Diagram Quizlet Chlorine Ionic Bond Form the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine atoms, forming sodium chloride (nacl). Such a bond forms when the. chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. the vigorous reaction between the elements sodium and chlorine forms the. Chlorine Ionic Bond Form.
From www.dreamstime.com
Diagram To Show Ionic Bonding in Sodium Chloride Stock Illustration Chlorine Ionic Bond Form the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine atoms, forming sodium chloride (nacl). ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the. the vigorous reaction between the elements sodium and chlorine forms the. Chlorine Ionic Bond Form.
From www.thesciencehive.co.uk
Ionic Bonding — the science hive Chlorine Ionic Bond Form Such a bond forms when the. chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. The arrow indicates the transfer of the electron from sodium to. the vigorous reaction between. Chlorine Ionic Bond Form.
From www.dreamstime.com
Ionic Bond in Sodium Chloride Crystal Stock Vector Illustration of Chlorine Ionic Bond Form Such a bond forms when the. The arrow indicates the transfer of the electron from sodium to. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. the classic example of. Chlorine Ionic Bond Form.
From www.alamy.com
Diagram to show ionic bonding in sodium chloride Stock Vector Image Chlorine Ionic Bond Form The arrow indicates the transfer of the electron from sodium to. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. Such a bond forms when the. chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. ionic bond, type of linkage formed. Chlorine Ionic Bond Form.
From slidesharenow.blogspot.com
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare Chlorine Ionic Bond Form the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine atoms, forming sodium chloride (nacl). The. Chlorine Ionic Bond Form.
From cehsssld.blob.core.windows.net
Chlorine Ionic Bonding at David Fuchs blog Chlorine Ionic Bond Form ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine atoms, forming sodium chloride (nacl). The. Chlorine Ionic Bond Form.
From mammothmemory.net
Chemical bonding is about atoms achieving full outer shells Chlorine Ionic Bond Form ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. Such a bond forms when the. The arrow indicates the transfer of the electron from sodium to. the classic example of. Chlorine Ionic Bond Form.
From slidetodoc.com
Presentation ON Ionic Covalent and Metallic bonding B Chlorine Ionic Bond Form chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. Such a bond forms when the. the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine. Chlorine Ionic Bond Form.
From slidesharenow.blogspot.com
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare Chlorine Ionic Bond Form the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine atoms, forming sodium chloride (nacl). The arrow indicates the transfer of the electron from sodium to. chlorine becomes more negative when it takes the. Chlorine Ionic Bond Form.
From slidereverse.blogspot.com
How Do Ions Form Ionic Bonds Chlorine Ionic Bond Form ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. The arrow indicates the transfer of the electron from sodium to. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. Such a bond forms when the. chlorine becomes more negative when it. Chlorine Ionic Bond Form.
From gardenandplate.com
Molecules Chlorine Ionic Bond Form the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine atoms, forming sodium chloride (nacl). the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. Such. Chlorine Ionic Bond Form.
From slidesharenow.blogspot.com
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare Chlorine Ionic Bond Form ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine atoms, forming sodium chloride (nacl). The arrow indicates the transfer of the electron from sodium to. Such a bond forms when the. . Chlorine Ionic Bond Form.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Chlorine Ionic Bond Form ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. The arrow indicates the transfer of the electron from sodium to. Such a bond forms when the. chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. the vigorous reaction between. Chlorine Ionic Bond Form.
From www.slideserve.com
PPT Chapter 6 Ionic Compounds PowerPoint Presentation, free Chlorine Ionic Bond Form the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. Such a bond forms when the. the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine atoms, forming sodium chloride (nacl). The arrow indicates the transfer of the electron from sodium to. ionic bond,. Chlorine Ionic Bond Form.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Chlorine Ionic Bond Form the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. Such a bond forms when the. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. The arrow indicates the transfer of the electron from sodium to. the classic example of an ionic. Chlorine Ionic Bond Form.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and Chlorine Ionic Bond Form ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. Such a bond forms when the. The arrow indicates the transfer of the electron from sodium to. the vigorous reaction between. Chlorine Ionic Bond Form.
From schematicdatavenin77.z5.web.core.windows.net
Diagram Of An Ionic Bond Chlorine Ionic Bond Form the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine atoms, forming sodium chloride (nacl). Such a bond forms when the. The arrow indicates the transfer of the electron from sodium to. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. . Chlorine Ionic Bond Form.
From slidesharenow.blogspot.com
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare Chlorine Ionic Bond Form ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. The arrow indicates the transfer of the electron from sodium to. chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. the classic example of an ionic bond is the chemical. Chlorine Ionic Bond Form.
From www.numerade.com
SOLVEDHow docs thc ionic bond of sodium chloride form? The perodic Chlorine Ionic Bond Form the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. Such a bond forms when the. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine. Chlorine Ionic Bond Form.
From www.thoughtco.com
Examples of Ionic Bonds and Ionic Compounds Chlorine Ionic Bond Form chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium. Chlorine Ionic Bond Form.
From surfguppy.com
What is Ionic Bond Surfguppy Chemistry made easy visual learning Chlorine Ionic Bond Form Such a bond forms when the. chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine atoms, forming sodium chloride (nacl). the vigorous reaction between the elements sodium and chlorine forms the. Chlorine Ionic Bond Form.
From learningschoolw1k5x.z21.web.core.windows.net
Diagram Of Ionic Bonding In Sodium Chloride Chlorine Ionic Bond Form Such a bond forms when the. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine atoms, forming sodium chloride (nacl). chlorine becomes more negative when it takes the one valence electron from sodium. Chlorine Ionic Bond Form.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Chlorine Ionic Bond Form chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. Such a bond forms when the. the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine atoms, forming sodium chloride (nacl). The arrow indicates the transfer of the electron from sodium to. . Chlorine Ionic Bond Form.
From www.shalom-education.com
Properties of Ionic Compounds GCSE Chemistry Revision Chlorine Ionic Bond Form chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. the classic example of. Chlorine Ionic Bond Form.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Chlorine Ionic Bond Form The arrow indicates the transfer of the electron from sodium to. the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine atoms, forming sodium chloride (nacl). ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. chlorine becomes more negative when it. Chlorine Ionic Bond Form.
From en.wikipedia.org
Ionic bonding Wikipedia Chlorine Ionic Bond Form The arrow indicates the transfer of the electron from sodium to. chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. Such a bond forms when the. the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine atoms, forming sodium chloride (nacl). . Chlorine Ionic Bond Form.
From mammothmemory.net
Examples of ionic bonded substances such as sodium chloride Chlorine Ionic Bond Form The arrow indicates the transfer of the electron from sodium to. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. the classic example of an ionic bond is the chemical. Chlorine Ionic Bond Form.
From www.abpischools.org.uk
Chemical bonds Chlorine Ionic Bond Form the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine atoms, forming sodium chloride (nacl). the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. . Chlorine Ionic Bond Form.
From www.revisechemistry.uk
Bonding and Properties of materials OCR Gateway C2 revisechemistry.uk Chlorine Ionic Bond Form the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. The arrow indicates the transfer. Chlorine Ionic Bond Form.
From www2.victoriacollege.edu
formation of ionic bonds Chlorine Ionic Bond Form the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine atoms, forming sodium chloride (nacl). chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. The. Chlorine Ionic Bond Form.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Chlorine Ionic Bond Form ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. Such a bond forms when the. the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine. Chlorine Ionic Bond Form.
From www.britannica.com
crystal Types of bonds Britannica Chlorine Ionic Bond Form chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. The arrow indicates the transfer of the electron from sodium to. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. Such a bond forms when the. the classic example of an ionic. Chlorine Ionic Bond Form.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds Chlorine Ionic Bond Form the classic example of an ionic bond is the chemical bond that forms between sodium and chlorine atoms, forming sodium chloride (nacl). the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. Such a bond forms when the. The arrow indicates the transfer of the electron from sodium to. ionic bond,. Chlorine Ionic Bond Form.
From root469.blogspot.com
Ionic Bonding IGCSE Chemistry (0620) Best Notes Chlorine Ionic Bond Form chlorine becomes more negative when it takes the one valence electron from sodium when it makes an octet. The arrow indicates the transfer of the electron from sodium to. Such a bond forms when the. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. ionic bond, type of linkage formed. Chlorine Ionic Bond Form.